Chlamydia trachomatis glyceraldehyde 3-phosphate dehydrogenase: Enzyme kinetics, high-resolution crystal structure, and plasminogen binding
- PMID: 33058314
- PMCID: PMC7679969
- DOI: 10.1002/pro.3975
Chlamydia trachomatis glyceraldehyde 3-phosphate dehydrogenase: Enzyme kinetics, high-resolution crystal structure, and plasminogen binding
Abstract
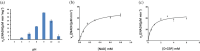
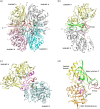
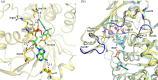
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is an evolutionarily conserved essential enzyme in the glycolytic pathway. GAPDH is also involved in a wide spectrum of non-catalytic cellular 'moonlighting' functions. Bacterial surface-associated GAPDHs engage in many host interactions that aid in colonization, pathogenesis, and virulence. We have structurally and functionally characterized the recombinant GAPDH of the obligate intracellular bacteria Chlamydia trachomatis, the leading cause of sexually transmitted bacterial and ocular infections. Contrary to earlier speculations, recent data confirm the presence of glucose-catabolizing enzymes including GAPDH in both stages of the biphasic life cycle of the bacterium. The high-resolution crystal structure described here provides a close-up view of the enzyme's active site and surface topology and reveals two chemically modified cysteine residues. Moreover, we show for the first time that purified C. trachomatis GAPDH binds to human plasminogen and plasmin. Based on the versatility of GAPDH's functions, data presented here emphasize the need for investigating the Chlamydiae GAPDH's involvement in biological functions beyond energy metabolism.
Keywords: Chlamydia; GAPDH; STD/STI; crystal structure; enzyme kinetics; glycolysis; plasmin binding; plasminogen binding; protein-protein interaction; reactive cysteine.
© 2020 The Protein Society.
Conflict of interest statement
The authors declare that they have no conflict of interest with the content of this article.
Figures
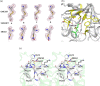

Similar articles
-
Structures of glyceraldehyde 3-phosphate dehydrogenase in Neisseria gonorrhoeae and Chlamydia trachomatis.Protein Sci. 2020 Mar;29(3):768-778. doi: 10.1002/pro.3824. Epub 2020 Jan 28. Protein Sci. 2020. PMID: 31930578 Free PMC article.
-
Structure of Streptococcus agalactiae glyceraldehyde-3-phosphate dehydrogenase holoenzyme reveals a novel surface.Acta Crystallogr F Struct Biol Commun. 2014 Oct;70(Pt 10):1333-9. doi: 10.1107/S2053230X14019517. Epub 2014 Sep 25. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 25286935 Free PMC article.
-
Cloning, expression, purification, crystallization and preliminary X-ray diffraction analysis of glyceraldehyde-3-phosphate dehydrogenase from Streptococcus agalactiae NEM316.Acta Crystallogr F Struct Biol Commun. 2014 Jul;70(Pt 7):938-41. doi: 10.1107/S2053230X14011418. Epub 2014 Jun 18. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 25005093 Free PMC article.
-
GAPDH as a model non-canonical AU-rich RNA binding protein.Semin Cell Dev Biol. 2019 Feb;86:162-173. doi: 10.1016/j.semcdb.2018.03.013. Epub 2018 Mar 24. Semin Cell Dev Biol. 2019. PMID: 29574117 Review.
-
Diverse Localization and Protein Binding Abilities of Glyceraldehyde-3-Phosphate Dehydrogenase in Pathogenic Bacteria: The Key to its Multifunctionality?Front Cell Infect Microbiol. 2020 Mar 3;10:89. doi: 10.3389/fcimb.2020.00089. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32195198 Free PMC article. Review.
Cited by
-
The role of tryptophan in Chlamydia trachomatis persistence.Front Cell Infect Microbiol. 2022 Aug 2;12:931653. doi: 10.3389/fcimb.2022.931653. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35982780 Free PMC article. Review.
-
Comprehensive Analysis of Alteration Landscape and Its Clinical Significance of Mitochondrial Energy Metabolism Pathway-Related Genes in Lung Cancers.Oxid Med Cell Longev. 2021 Dec 20;2021:9259297. doi: 10.1155/2021/9259297. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34970420 Free PMC article.
-
Identification of the Glyceraldehyde-3-Phosphate Dehydrogenase (GeGAPDH) Gene Family in Gastrodia elata Revealing Its Response Characteristics to Low-Temperature and Pathogen Stress.Plants (Basel). 2025 Jun 18;14(12):1866. doi: 10.3390/plants14121866. Plants (Basel). 2025. PMID: 40573855 Free PMC article.
-
Glyceraldehyde 3-Phosphate Dehydrogenase on the Surface of Candida albicans and Nakaseomyces glabratus Cells-A Moonlighting Protein That Binds Human Vitronectin and Plasminogen and Can Adsorb to Pathogenic Fungal Cells via Major Adhesins Als3 and Epa6.Int J Mol Sci. 2024 Jan 13;25(2):1013. doi: 10.3390/ijms25021013. Int J Mol Sci. 2024. PMID: 38256088 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

