Catalysis with Palladium(I) Dimers
- PMID: 33058375
- PMCID: PMC7898807
- DOI: 10.1002/anie.202011825
Catalysis with Palladium(I) Dimers
Abstract
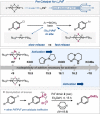
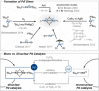
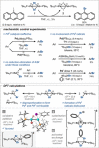
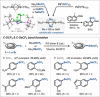
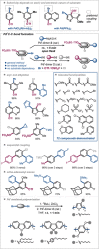
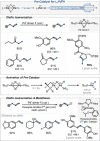
Dinuclear PdI complexes have found widespread applications as diverse catalysts for a multitude of transformations. Initially their ability to function as pre-catalysts for low-coordinated Pd0 species was harnessed in cross-coupling. Such PdI dimers are inherently labile and relatively sensitive to oxygen. In recent years, more stable dinuclear PdI -PdI frameworks, which feature bench-stability and robustness towards nucleophiles as well as recoverability in reactions, were explored and shown to trigger privileged reactivities via dinuclear catalysis. This includes the predictable and substrate-independent, selective C-C and C-heteroatom bond formations of poly(pseudo)halogenated arenes as well as couplings of arenes with relatively weak nucleophiles, which would not engage in Pd0 /PdII catalysis. This Minireview highlights the use of dinuclear PdI complexes as both pre-catalysts for the formation of highly active Pd0 and PdII -H species as well as direct dinuclear catalysts. Focus is set on the mechanistic intricacies, the speciation and the impacts on reactivity.
Keywords: catalysis; cross-coupling; dinuclear PdI; palladium; pre-catalyst.
© 2020 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- None
-
- New Trends in Cross-Coupling : Theory and Applications (Ed.: Colacot T. J.), The Royal Society of Chemistry, Cambridge, 2015;
-
- Powers I. G., Uyeda C., ACS Catal. 2017, 7, 936–958;
-
- Lee C. L., James B. R., Nelson D. A., Hallen R. T., Organometallics 1984, 3, 1360–1364.
Publication types
LinkOut - more resources
Full Text Sources

