A Therapeutic Non-self-reactive SARS-CoV-2 Antibody Protects from Lung Pathology in a COVID-19 Hamster Model
- PMID: 33058755
- PMCID: PMC7510528
- DOI: 10.1016/j.cell.2020.09.049
A Therapeutic Non-self-reactive SARS-CoV-2 Antibody Protects from Lung Pathology in a COVID-19 Hamster Model
Abstract
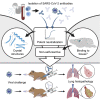
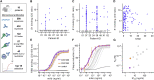
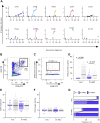
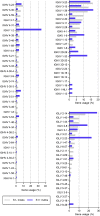
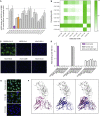
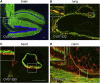
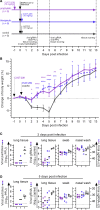
The emergence of SARS-CoV-2 led to pandemic spread of coronavirus disease 2019 (COVID-19), manifesting with respiratory symptoms and multi-organ dysfunction. Detailed characterization of virus-neutralizing antibodies and target epitopes is needed to understand COVID-19 pathophysiology and guide immunization strategies. Among 598 human monoclonal antibodies (mAbs) from 10 COVID-19 patients, we identified 40 strongly neutralizing mAbs. The most potent mAb, CV07-209, neutralized authentic SARS-CoV-2 with an IC50 value of 3.1 ng/mL. Crystal structures of two mAbs in complex with the SARS-CoV-2 receptor-binding domain at 2.55 and 2.70 Å revealed a direct block of ACE2 attachment. Interestingly, some of the near-germline SARS-CoV-2-neutralizing mAbs reacted with mammalian self-antigens. Prophylactic and therapeutic application of CV07-209 protected hamsters from SARS-CoV-2 infection, weight loss, and lung pathology. Our results show that non-self-reactive virus-neutralizing mAbs elicited during SARS-CoV-2 infection are a promising therapeutic strategy.
Keywords: COVID-19; SARS-CoV-2; autoreactivity; crystal structures; hamster model; monoclonal antibody; neutralizing antibody; post-exposure; self-antigens; self-reactivity.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests Related to this work, the German Center for Neurodegenerative Diseases (DZNE) and Charité-Universitätsmedizin Berlin have filed a patent application on which J.K., S.M.R., H.-C.K., E.S.-S., V.M.C., M.A.M., D.W., L.E.S., and H.P. are named as inventors.
Figures


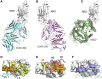


Update of
-
A SARS-CoV-2 neutralizing antibody protects from lung pathology in a COVID-19 hamster model.bioRxiv [Preprint]. 2020 Aug 16:2020.08.15.252320. doi: 10.1101/2020.08.15.252320. bioRxiv. 2020. Update in: Cell. 2020 Nov 12;183(4):1058-1069.e19. doi: 10.1016/j.cell.2020.09.049. PMID: 32817952 Free PMC article. Updated. Preprint.
Comment in
-
'nAb' the self-reactive activity in the COVID-19 combat.Signal Transduct Target Ther. 2021 Mar 2;6(1):105. doi: 10.1038/s41392-021-00518-2. Signal Transduct Target Ther. 2021. PMID: 33654054 Free PMC article. No abstract available.
References
-
- Barnes C.O., West A.P., Jr., Huey-Tubman K.E., Hoffmann M.A.G., Sharaf N.G., Hoffman P.R., Koranda N., Gristick H.B., Gaebler C., Muecksch F. Structures of Human Antibodies Bound to SARS-CoV-2 Spike Reveal Common Epitopes and Recurrent Features of Antibodies. Cell. 2020;182:828–842.e16. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

