Tetracycline Antibiotics Induce Host-Dependent Disease Tolerance to Infection
- PMID: 33058782
- PMCID: PMC7840524
- DOI: 10.1016/j.immuni.2020.09.011
Tetracycline Antibiotics Induce Host-Dependent Disease Tolerance to Infection
Abstract
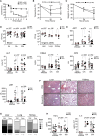
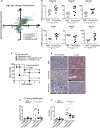
Several classes of antibiotics have long been known to have beneficial effects that cannot be explained strictly on the basis of their capacity to control the infectious agent. Here, we report that tetracycline antibiotics, which target the mitoribosome, protected against sepsis without affecting the pathogen load. Mechanistically, we found that mitochondrial inhibition of protein synthesis perturbed the electron transport chain (ETC) decreasing tissue damage in the lung and increasing fatty acid oxidation and glucocorticoid sensitivity in the liver. Using a liver-specific partial and acute deletion of Crif1, a critical mitoribosomal component for protein synthesis, we found that mice were protected against sepsis, an observation that was phenocopied by the transient inhibition of complex I of the ETC by phenformin. Together, we demonstrate that mitoribosome-targeting antibiotics are beneficial beyond their antibacterial activity and that mitochondrial protein synthesis inhibition leading to ETC perturbation is a mechanism for the induction of disease tolerance.
Keywords: disease tolerance; doxycycline; electron transport chain; immunometabolism; liver; lung; mitochondria; mitoribosome; sepsis.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





Comment in
-
Antibiotic-induced disease tolerance.Nat Rev Immunol. 2020 Dec;20(12):717. doi: 10.1038/s41577-020-00469-9. Nat Rev Immunol. 2020. PMID: 33077934 No abstract available.
-
Targeting Bacteria within Us to Diminish Infection and Autoimmunity.Immunity. 2021 Jan 12;54(1):1-3. doi: 10.1016/j.immuni.2020.12.006. Immunity. 2021. PMID: 33440134
-
Disease tolerance to infection: the immune defense strategy of mitoribosome targeting.Cell Mol Immunol. 2021 Jul;18(7):1626-1627. doi: 10.1038/s41423-021-00677-w. Epub 2021 Apr 16. Cell Mol Immunol. 2021. PMID: 33864005 Free PMC article. No abstract available.
References
-
- Aksoy M.O., Mardini I.A., Yang Y., Bin W., Zhou S., Kelsen S.G. Glucocorticoid effects on the β-adrenergic receptor-adenylyl cyclase system of human airway epithelium. J. Allergy Clin. Immunol. 2002;109:491–497. - PubMed
-
- Cecconi M., Evans L., Levy M., Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75–87. - PubMed

