Papain-like cysteine proteinase zone (PCP-zone) and PCP structural catalytic core (PCP-SCC) of enzymes with cysteine proteinase fold
- PMID: 33058970
- PMCID: PMC7548629
- DOI: 10.1016/j.ijbiomac.2020.10.022
Papain-like cysteine proteinase zone (PCP-zone) and PCP structural catalytic core (PCP-SCC) of enzymes with cysteine proteinase fold
Abstract
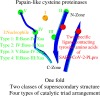
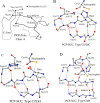
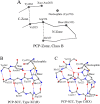
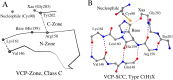
There are several families of cysteine proteinases with different folds - for example the (chymo)trypsin fold family and papain-like fold family - but in both families the hydrolase activity of cysteine proteinases requires a cysteine residue as the catalytic nucleophile. In this work, we have analyzed the topology of the active site regions in 146 three-dimensional structures of proteins belonging to the Papain-like Cysteine Proteinase (PCP) superfamily, which includes papain as a typical representative of this protein superfamily. All analyzed enzymes contain a unique structurally closed conformation - a "PCP-Zone" - which can be divided into two groups, Class A and Class B. Eight structurally conserved amino acids of the PCP-Zone form a common Structural Core. The Structural Core, catalytic nucleophile, catalytic base and residue Xaa - which stabilizes the side-chain conformation of the catalytic base - make up a PCP Structural Catalytic Core (PCP-SCC). The PCP-SCC of Class A and Class B are divided into 5 and 2 types, respectively. Seven variants of the mutual arrangement of the amino-acid side chains of the catalytic triad - nucleophile, base and residue Xaa - within the same fold clearly demonstrate how enzymes with the papain-like fold adapt to the need to perform diverse functions in spite of their limited structural diversity. The roles of both the PCP-Zone of SARS-CoV-2-PLpro described in this study and the NBCZone of SARS-CoV-2-3CLpro presented in our earlier article (Denesyuk AI, Johnson MS, Salo-Ahen OMH, Uversky VN, Denessiouk K. Int J Biol Macromol. 2020;153:399-411) that are in contacts with inhibitors are discussed.
Keywords: COVID-19; Catalytic triad; Cysteine proteinases; Fold; Papain; SARC-CoV-2; Structural catalytic core; Zone.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest.
Figures
References
-
- Dodson G., Wlodawer A. Catalytic triads and their relatives. Trends Biochem. Sci. 1998;23(9):347–352. - PubMed
-
- Denesyuk A.I., Johnson M.S., Salo-Ahen O.M.H., Uversky V.N., Denessiouk K. NBCZone: universal three-dimensional construction of eleven amino acids near the catalytic nucleophile and base in the superfamily of (chymo)trypsin-like serine fold proteases. Int. J. Biol. Macromol. 2020;153:399–411. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

