Immune Profiling Enables Stratification of Patients With Active Tuberculosis Disease or Mycobacterium tuberculosis Infection
- PMID: 33059361
- PMCID: PMC8563210
- DOI: 10.1093/cid/ciaa1562
Immune Profiling Enables Stratification of Patients With Active Tuberculosis Disease or Mycobacterium tuberculosis Infection
Abstract
Background: Tuberculosis (TB) is caused by Mycobacterium tuberculosis (Mtb) infection and is a major public health problem. Clinical challenges include the lack of a blood-based test for active disease. Current blood-based tests, such as QuantiFERON (QFT) do not distinguish active TB disease from asymptomatic Mtb infection.
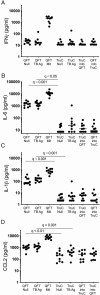
Methods: We hypothesized that TruCulture, an immunomonitoring method for whole-blood stimulation, could discriminate active disease from latent Mtb infection (LTBI). We stimulated whole blood from patients with active TB and compared with LTBI donors. Mtb-specific antigens and live bacillus Calmette-Guérin (BCG) were used as stimuli, with direct comparison to QFT. Protein analyses were performed using conventional and digital enzyme-linked immunosorbent assay (ELISA), as well as Luminex.
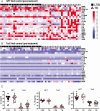
Results: TruCulture showed discrimination of active TB cases from LTBI (P < .0001, AUC = .81) compared with QFT (P = .45, AUC = .56), based on an interferon γ (IFNγ) readout after Mtb antigen (Ag) stimulation. This result was replicated in an independent cohort (AUC = .89). In exploratory analyses, TB stratification could be further improved by the Mtb antigen to BCG IFNγ ratio (P < .0001, AUC = .91). Finally, the combination of digital ELISA and transcriptional analysis showed that LTBI donors with high IFNγ clustered with patients with TB, suggesting the possibility to identify subclinical disease.
Conclusions: TruCulture offers a next-generation solution for whole-blood stimulation and immunomonitoring with the possibility to discriminate active and latent infection.
Keywords: biomarkers; cytokines; immune profiling; patient stratification; tuberculosis.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures




References
-
- World Health Organization. Global tuberculosis report 2018. Geneva, Switzerland, World Health Organization, 2018.
-
- O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol 2013; 31:475–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

