Evaluation of hepatic fibrosis in HIV/HCV co-infected individuals in Yaoundé, Cameroon: usefulness of APRI score in resource-constrained settings
- PMID: 33059627
- PMCID: PMC7558964
- DOI: 10.1186/s12879-020-05477-7
Evaluation of hepatic fibrosis in HIV/HCV co-infected individuals in Yaoundé, Cameroon: usefulness of APRI score in resource-constrained settings
Abstract
Background: HIV infection exacerbates the prognosis of HCV infection, with a faster progression of hepatitis. Hepatic fibrosis is the major disruption of the hepatic tissue architecture characterized by anarchic deposition and excess of the extracellular matrix. The objective of this study was to evaluate hepatic fibrosis in HIV/HCV co-infected individuals as compared to HCV mono-infected.
Methods: A total of 97 participants (mean age 60.2 ± 14.3 years and 0.76 male/female sex ratio) was enrolled in a study conducted in Yaoundé, Cameroon from November 2018 to January 2019. Liver fibrosis was assessed by the APRI score (Aspartate Aminotransferase or AST/Platelet Ratio Index) which identifies the stage of fibrosis as classified by the Metavir system (F0 to F4). CD4 counts and plasmatic HIV viral load of HIV/HCV co-infected individuals were determined and the correlation between hepatic fibrosis and immuno-virological status established. Statistical analysis was done using Microsoft Excel 2016 and EpiInfo7 software.
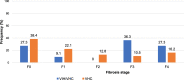
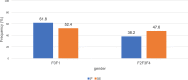
Results: A high proportion (63.6%) of HIV/HCV co-infected participants had an abnormal AST level: 73.6 ± 45.8 IU/L as compared to 58.5 ± 39.3 IU/L (59.3%) among HCV mono-infected participants. The frequency of thrombocytopenia was 63.6% with a mean platelet count of 137 ± 50 × 103 IU/L in HIV/HCV co-infected participants as compared to 176 ± 67 × 103 IU/L in HCV mono-infected participants (38.4%). The progression of hepatic fibrosis in participants with clinically significant fibrosis: F2, F3 and F4 was higher among HIV/HCV co-infected and the mean APRI score was 1.7 ± 1.4 versus 1 ± 0.8 among HCV mono-infected (26.7%). All participants (100%) with detectable HIV viral load had clinically significant fibrosis compared to 33.4% in those with undetectable HIV viral load (p = 0.55). Only 42.9% participants with CD4 > 500 cells/μL had clinically significant fibrosis (p = 0.72) while 100% participants with CD4 < 200 cells/μL had clinically significant fibrosis (p = 0.58).
Conclusions: A high level of AST combined with thrombocytopenia (APRI score > 1.5) is an indicator of hepatic fibrosis in HIV/HCV co-infected individuals. Because of its non-invasive and less costly nature, the APRI score can be a suitable biomarker to monitor hepatic fibrosis in HIV/HCV co-infected individuals in resource constrained settings.
Keywords: APRI score; HIV/HCV co-infection; Liver fibrosis; Resource constrained settings; Thrombocytopenia.
Conflict of interest statement
The authors declared that, this study is without conflicts of interests.
Figures
Similar articles
-
Cocaine/crack use is not associated with fibrosis progression measured by AST-to-Platelet Ratio Index in HIV-HCV co-infected patients: a cohort study.BMC Infect Dis. 2017 Jan 17;17(1):80. doi: 10.1186/s12879-017-2196-0. BMC Infect Dis. 2017. PMID: 28095797 Free PMC article.
-
Hepatic Fibrosis Progression in HIV-Hepatitis C Virus Co-Infection--The Effect of Sex on Risk of Significant Fibrosis Measured by Aspartate-to-Platelet Ratio Index.PLoS One. 2015 Jun 19;10(6):e0129868. doi: 10.1371/journal.pone.0129868. eCollection 2015. PLoS One. 2015. PMID: 26090666 Free PMC article.
-
Elevated AST-to-platelet ratio index is associated with increased all-cause mortality among HIV-infected adults in Zambia.Liver Int. 2015 Jul;35(7):1886-92. doi: 10.1111/liv.12780. Epub 2015 Jan 22. Liver Int. 2015. PMID: 25581487 Free PMC article.
-
Analysis of noninvasive methods in chronic hepatitis/human immunodeficiency virus mono- and co-infected patients with advanced fibrosis.Eur J Gastroenterol Hepatol. 2025 May 1;37(5):638-643. doi: 10.1097/MEG.0000000000002936. Epub 2025 Feb 6. Eur J Gastroenterol Hepatol. 2025. PMID: 39976004
-
The involvement of microRNAs in HCV and HIV infection.Ther Adv Vaccines Immunother. 2022 Jul 5;10:25151355221106104. doi: 10.1177/25151355221106104. eCollection 2022. Ther Adv Vaccines Immunother. 2022. PMID: 35832725 Free PMC article. Review.
Cited by
-
Sofosbuvir plus Ribavirin is effective for HCV elimination in people living with HIV from rural area of China.Sci Rep. 2021 May 28;11(1):11301. doi: 10.1038/s41598-021-90706-5. Sci Rep. 2021. PMID: 34050222 Free PMC article.
-
Indirect serum biomarkers perform sub optimally in screening for significant liver fibrosis among HIV-infected and uninfected adults in Uganda.Afr Health Sci. 2022 Sep;22(3):416-425. doi: 10.4314/ahs.v22i3.45. Afr Health Sci. 2022. PMID: 36910378 Free PMC article.
-
Clinical significances of liver fibrotic markers in patients with cholangiocarcinoma after radical resections.Turk J Surg. 2024 Dec 27;40(4):283-295. doi: 10.47717/turkjsurg.2024.6486. eCollection 2024 Dec. Turk J Surg. 2024. PMID: 39980645 Free PMC article.
-
HIV-Infected Hepatic Stellate Cells or HCV-Infected Hepatocytes Are Unable to Promote Latency Reversal among HIV-Infected Mononuclear Cells.Pathogens. 2024 Feb 1;13(2):134. doi: 10.3390/pathogens13020134. Pathogens. 2024. PMID: 38392872 Free PMC article.
References
-
- UNAIDS . 2019 Global HIV Statistics. 2020.
-
- Aubry PP, Gaüzère B. Infection par le VIH/Sida et tropiques. Mise à jour le 08/07/2018. 2018.
-
- Organisation . Mondiale de la Sante Rapport mondiale sur l'hepatite, 2017. 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

