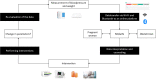
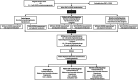
Design of the Pregnancy REmote MOnitoring II study (PREMOM II): a multicenter, randomized controlled trial of remote monitoring for gestational hypertensive disorders
- PMID: 33059633
- PMCID: PMC7565319
- DOI: 10.1186/s12884-020-03291-2
Design of the Pregnancy REmote MOnitoring II study (PREMOM II): a multicenter, randomized controlled trial of remote monitoring for gestational hypertensive disorders
Abstract
Background: Observational data from the retrospective, non-randomized Pregnancy REmote MOnitoring I (PREMOM I) study showed that remote monitoring (RM) may be beneficial for prenatal observation of women at risk for gestational hypertensive disorders (GHD) in terms of clinical outcomes, health economics, and stakeholder perceptions. PREMOM II is a prospective, randomized, multicenter follow-up study that was performed to explore these promising results.
Methods: After providing written consent, 3922 pregnant women aged ≥18 years who are at increased risk of developing GHD will be randomized (1:1:1 ratio) to (a) conventional care (control group), (b) a patient self-monitoring group, and (c) a midwife-assisted RM group. The women in each group will be further divided (1:1 ratio) to evaluate the outcomes of targeted or non-targeted (conventional) antihypertensive medication. Women will be recruited in five hospitals in Flanders, Belgium: Ziekenhuis Oost-Limburg, Universitaire Ziekenhuis Antwerpen, Universitaire Ziekenhuis Leuven, AZ Sint Jan Brugge-Oostende, and AZ Sint Lucas Brugge. The primary outcomes are: (1) numbers and types of prenatal visits; (2) maternal outcomes; (3) neonatal outcomes; (4) the applicability and performance of RM; and (5) compliance with RM and self-monitoring. The secondary outcomes are: (1) cost-effectiveness and willingness to pay; (2) patient-reported outcome measures (PROMS) questionnaires on the experiences of the participants; and (3) the maternal and perinatal outcomes according to the type of antihypertensive medication. Demographic, and maternal and neonatal outcomes are collected from the patients' electronic records. Blood pressure and compliance rate will be obtained from an online digital coordination platform for remote data handling. Information about the healthcare-related costs will be obtained from the National Coordination Committee of Belgian Health Insurance Companies (Intermutualistisch Agentschap). PROMS will be assessed using validated questionnaires.
Discussion: To our knowledge, this is the first randomized trial comparing midwife-assisted RM and self-monitoring of prenatal blood pressure versus conventional management among women at increased risk of GHD. Positive results of this study may lead to a practical framework for caregivers, hospital management, and payers to introduce RM into the prenatal care programs of high-risk pregnancies.
Trial registration: This study was registered on clinicaltrials.gov , identification number NCT04031430. Registered 24 July 2019, https://clinicaltrials.gov/ct2/show/NCT04031430?cond=premom+ii&draw=2&rank=1 .
Keywords: Gestational hypertensive disorders; Pre-eclampsia; Remote monitoring.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Devlieger R, Martens E, Martens G, Van Mol C, Cammu H. Perinatale activiteiten in Vlaanderen 2015. Brussel: SPE; 2016.
-
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. 2020. http://www.acog.org/Resources-And-Publications/TaskForce-and-Work-Group-.... Accessed 22 July 2020.
-
- Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2014;2:CD002252. - PubMed
-
- Magee LA, Duley L. Oral beta-blockers for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2003;3:CD002863. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical