Forsythoside B attenuates memory impairment and neuroinflammation via inhibition on NF-κB signaling in Alzheimer's disease
- PMID: 33059746
- PMCID: PMC7565774
- DOI: 10.1186/s12974-020-01967-2
Forsythoside B attenuates memory impairment and neuroinflammation via inhibition on NF-κB signaling in Alzheimer's disease
Abstract
Background: Neuroinflammation is a principal element in Alzheimer's disease (AD) pathogenesis, so anti-inflammation may be a promising therapeutic strategy. Forsythoside B (FTS•B), a phenylethanoid glycoside isolated from Forsythiae fructus, has been reported to exert anti-inflammatory effects. However, no studies have reported whether the anti-inflammatory properties of FTS•B have a neuroprotective effect in AD. In the present study, these effects of FTS•B were investigated using amyloid precursor protein/presenilin 1 (APP/PS1) mice, BV-2 cells, and HT22 cells.
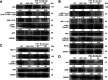
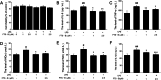
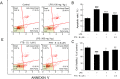
Methods: APP/PS1 mice were administered FTS•B intragastrically for 36 days. Behavioral tests were then carried out to examine cognitive functions, including the Morris water maze, Y maze, and open field experiment. Immunohistochemistry was used to analyze the deposition of amyloid-beta (Aβ), the phosphorylation of tau protein, and the levels of 4-hydroxynonenal, glial fibrillary acidic protein, and ionized calcium-binding adapter molecule 1 in the hippocampus. Proteins that showed marked changes in levels related to neuroinflammation were identified using proteomics and verified using enzyme-linked immunosorbent assay and western blot. BV-2 and HT22 cells were also used to confirm the anti-neuroinflammatory effects of FTS•B.
Results: In APP/PS1 mice, FTS•B counteracted cognitive decline, ameliorated the deposition of Aβ and the phosphorylation of tau protein, and attenuated the activation of microglia and astrocytes in the cortex and hippocampus. FTS•B affected vital signaling, particularly by decreasing the activation of JNK-interacting protein 3/C-Jun NH2-terminal kinase and suppressing WD-repeat and FYVE-domain-containing protein 1/toll-like receptor 3 (WDFY1/TLR3), further suppressing the activation of nuclear factor-κB (NF-κB) signaling. In BV-2 and HT22 cells, FTS•B prevented lipopolysaccharide-induced neuroinflammation and reduced the microglia-mediated neurotoxicity.
Conclusions: FTS•B effectively counteracted cognitive decline by regulating neuroinflammation via NF-κB signaling in APP/PS1 mice, providing preliminary experimental evidence that FTS•B is a promising therapeutic agent in AD treatment.
Keywords: Alzheimer’s disease; Forsythoside B; NF-κB; Neuroinflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Bessey LJ, Walaszek A. Management of behavioral and psychological symptoms of dementia. Current Psychiatry Reports. 2019;21. - PubMed
-
- Medrano-Jimenez E, Jimenez-Ferrer Carrillo I, Pedraza-Escalona M, Ramirez-Serrano CE, Alvarez-Arellano L, Cortes-Mendoza J, Herrera-Ruiz M, Jimenez-Ferrer E, Zamilpa A, Tortoriello J, et al. Malva parviflora extract ameliorates the deleterious effects of a high fat diet on the cognitive deficit in a mouse model of Alzheimer’s disease by restoring microglial function via a PPAR-gamma-dependent mechanism. J Neuroinflammation. 2019;16. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2018YFE0107800/Ministry of Science and Technology of the People's Republic of China
- JJKH20190108KJ/"13th Five-year" Science and Technology Projects from Education Department in Jilin Province of P. R. China
- 2019C050-8/Industrial Technology Research and Development Projects from Development and Reform Commission of Jilin Province
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

