Challenges in Cardiac and Pulmonary Sarcoidosis: JACC State-of-the-Art Review
- PMID: 33059834
- PMCID: PMC7808240
- DOI: 10.1016/j.jacc.2020.08.042
Challenges in Cardiac and Pulmonary Sarcoidosis: JACC State-of-the-Art Review
Abstract
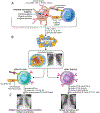
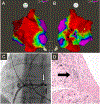
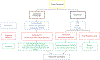
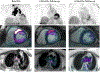
Sarcoidosis is a complex disease with heterogeneous clinical presentations that can affect virtually any organ. Although the lung is typically the most common organ involved, combined pulmonary and cardiac sarcoidosis (CS) account for most of the morbidity and mortality associated with this disease. Pulmonary sarcoidosis can be asymptomatic or result in impairment in quality of life and end-stage, severe, and/or life-threatening disease. The latter outcome is seen almost exclusively in those with fibrotic pulmonary sarcoidosis, which accounts for 10% to 20% of pulmonary sarcoidosis patients. CS is problematic to diagnose and may cause significant morbidity and death from heart failure or ventricular arrhythmias. The diagnosis of CS usually requires surrogate cardiac imaging biomarkers, as endomyocardial biopsy has relatively low yield, even with directed electrophysiological mapping. Treatment of CS is often multifactorial, involving a combination of antigranulomatous therapy and pharmacotherapy for cardiac arrhythmias and/or heart failure in addition to device placement and cardiac transplantation.
Keywords: biomarkers; cardiac sarcoidosis; imaging; pulmonary sarcoidosis.
Copyright © 2020 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis 2012;29:119–27. - PubMed
-
- Morimoto T, Azuma A et al. Epidemiology of sarcoidosis in Japan. Eur Respir J 2008;31:372–9. - PubMed
-
- Ohta H, Tazawa R et al. Acute-onset sarcoidosis with erythema nodosum and polyarthralgia (Lofgren’s syndrome) in Japan: a case report and a review of the literature. Intern Med 2006;45:659–62. - PubMed
-
- Birnie DH, Nery PB et al. Cardiac Sarcoidosis. J Am Coll Cardiol 2016;68:411–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

