Controlling Horizontal Cell-Mediated Lateral Inhibition in Transgenic Zebrafish Retina with Chemogenetic Tools
- PMID: 33060180
- PMCID: PMC7665903
- DOI: 10.1523/ENEURO.0022-20.2020
Controlling Horizontal Cell-Mediated Lateral Inhibition in Transgenic Zebrafish Retina with Chemogenetic Tools
Abstract
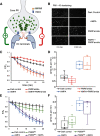
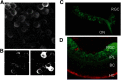
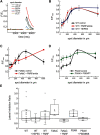
Horizontal cells (HCs) form reciprocal synapses with rod and cone photoreceptors, an arrangement that underlies lateral inhibition in the retina. HCs send negative and positive feedback signals to photoreceptors, but how HCs initiate these signals remains unclear. Unfortunately, because HCs have no unique neurotransmitter receptors, there are no pharmacological treatments for perturbing membrane potential specifically in HCs. Here we use transgenic zebrafish whose HCs express alien receptors, enabling cell-type-specific control by cognate alien agonists. To depolarize HCs, we used the Phe-Met-Arg-Phe-amide (FMRFamide)-gated Na+ channel (FaNaC) activated by the invertebrate neuropeptide FMRFamide. To hyperpolarize HCs we used a pharmacologically selective actuator module (PSAM)-glycine receptor (GlyR), an engineered Cl- selective channel activated by a synthetic agonist. Expression of FaNaC or PSAM-GlyR was restricted to HCs with the cell-type selective promoter for connexin-55.5. We assessed HC-feedback control of photoreceptor synapses in three ways. First, we measured presynaptic exocytosis from photoreceptor terminals using the fluorescent dye FM1-43. Second, we measured the electroretinogram (ERG) b-wave, a signal generated by postsynaptic responses. Third, we used Ca2+ imaging in retinal ganglion cells (RGCs) expressing the Ca2+ indicator GCaMP6. Addition of FMRFamide significantly decreased FM1-43 destaining in darkness, whereas the addition of PSAM-GlyR significantly increased it. However, both agonists decreased the light-elicited ERG b-wave and eliminated surround inhibition of the Ca2+ response of RGCs. Taken together, our findings show that chemogenetic tools can selectively manipulate negative feedback from HCs, providing a platform for understanding its mechanism and helping to elucidate its functional roles in visual information processing at a succession of downstream stages.
Keywords: feedback inhibition; fluorescent protein; horizontal cell; lateral inhibition; photoreceptor; retina.
Copyright © 2020 Beckwith-Cohen et al.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
