Secukinumab demonstrates high efficacy and a favorable safety profile over 52 weeks in Chinese patients with moderate to severe plaque psoriasis
- PMID: 33060370
- PMCID: PMC7647502
- DOI: 10.1097/CM9.0000000000001163
Secukinumab demonstrates high efficacy and a favorable safety profile over 52 weeks in Chinese patients with moderate to severe plaque psoriasis
Abstract
Background: Psoriasis is a chronic inflammatory skin disease, affecting about 0.6% of the Chinese population. Many patients are not well controlled by conventional treatments, thus there is need for new treatment regimens. In this study, we assessed the efficacy and safety of secukinumab in Chinese patients with moderate to severe plaque psoriasis.
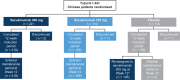
Methods: This study was a 52-week, multicentre, randomized, double-blind, placebo-controlled, parallel-group, Phase 3 trial. A sub-population of study participants (≥18 years) of Chinese ethnicity were randomized to receive subcutaneous injections of 300 or 150 mg secukinumab, or placebo. The co-primary endpoints were psoriasis area severity index (PASI) 75 and Investigator's Global Assessment (IGA) 0/1 at Week 12.
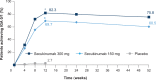
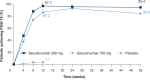
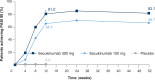
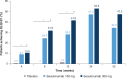
Results: A total of 441 Chinese patients were enrolled in this study. Co-primary outcomes were achieved; 300 and 150 mg secukinumab were superior to placebo as shown in the proportion of patients that achieved PASI 75 (97.7% and 87.2% vs. 3.7%, respectively; P < 0.001), and IGA 0/1 (82.3% and 69.7% vs. 2.7%; P < 0.001) at Week 12. Treatment efficacy was maintained until Week 52. There was no increase in overall adverse events with secukinumab relative to placebo throughout the 52-week period.
Conclusion: Secukinumab is highly effective and well tolerated in Chinese patients with moderate to severe plaque psoriasis.
Trial registration: ClinicalTrials.gov, NCT03066609; https://clinicaltrials.gov/ct2/show/record/NCT03066609.
Conflict of interest statement
Lin Cai has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from Novartis, AbbVie, Pfizer Inc. Jian-Zhong Zhang has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, La Roche-Posay China, AbbVie, Bayer, Janssen-Cilag, Henlius, Kyowa Kirin, and Pfizer Inc. Xu Yao has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, AbbVie, Bayer, Janssen-Cilag, and Pfizer Inc. Jun Gu has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, La Roche-Posay China, AbbVie, Bayer, Henlius, and Pfizer Inc. Quan-Zhong Liu has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from Novartis, La Roche-Posay China, AbbVie, Bayer, Janssen-Cilag, and Pfizer Inc. Min Zheng has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from AbbVie, Janssen-Cilag, Boehringer Ingelheim, LEO Pharma China, Xian-Janssen, Novartis, and Pfizer Inc. Shi-Fa Zhang has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Janssen-Cilag, Henlius. Jin-Hua Xu has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from Novartis, Sanofi, La Roche-Posay China, AbbVie, Bayer, Kyowa Kirin, and Pfizer Inc. Cheng-Xin Li has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, AbbVie, Bayer, Janssen-Cilag, Kyowa Kirin, and Pfizer Inc. Hao Cheng has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, AbbVie, Bayer, Janssen-Cilag, Henlius, and Pfizer Inc. Qing Guo has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, La Roche-Posay China, AbbVie, Bayer, Janssen-Cilag, Henlius, Kyowa Kirin, and Pfizer Inc. Wei-Li Pan has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, La Roche-Posay China, AbbVie, Bayer, Janssen-Cilag, Henlius, Kyowa Kirin, and Pfizer Inc. Shen-Qiu Li has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, AbbVie, Bayer, Janssen-Cilag, and Pfizer Inc. Ruo-Yu Li has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Bayer, Janssen-Cilag, MSD, and Pfizer Inc. Zai-Pei Guo has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, AbbVie, Bayer, Janssen-Cilag, and Pfizer Inc. Zhi-Qi Song has participated in advisory boards and/or as an investigator and/or speaker and received grants and/or honoraria from LEO Pharma China, Novartis, Sanofi, La Roche-Posay China, AbbVie, Bayer, Janssen-Cilag, Henlius, Kyowa Kirin, and Pfizer Inc. Shan-Shan Li has participated as an investigator and received honoraria from Novartis China. Xiu-Qin Dong has participated in advisory boards and/or as an investigator and/or speaker and received honoraria from LEO Pharma China, Novartis, Sanofi, AbbVie, Bayer, Janssen-Cilag. Linda Wang, Rong Fu, Pascaline Regnault, Pascal Charef, Rafal Mazur, and Manmath Patekar are employed by Novartis.
Figures
References
-
- Haroon M, Kirby B, FitzGerald O. High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann Rheum Dis 2013; 72:736–740. doi: 10.1136/annrheumdis-2012-201706. - PubMed
-
- Papadavid E, Katsimbri P, Kapniari I, Koumaki D, Karamparpa A, Dalamaga M, et al. Prevalence of psoriatic arthritis and its correlates among patients with psoriasis in Greece: results from a large retrospective study. J Eur Acad Dermatol Venereol 2016; 30:1749–1752. doi: 10.1111/jdv.13700. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

