Explosive-driven double-blast exposure: molecular, histopathological, and behavioral consequences
- PMID: 33060648
- PMCID: PMC7566442
- DOI: 10.1038/s41598-020-74296-2
Explosive-driven double-blast exposure: molecular, histopathological, and behavioral consequences
Abstract
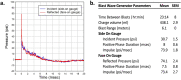
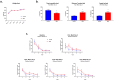
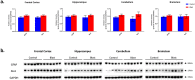
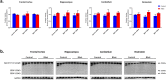
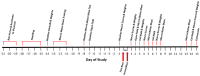
Traumatic brain injury generated by blast may induce long-term neurological and psychiatric sequelae. We aimed to identify molecular, histopathological, and behavioral changes in rats 2 weeks after explosive-driven double-blast exposure. Rats received two 30-psi (~ 207-kPa) blasts 24 h apart or were handled identically without blast. All rats were behaviorally assessed over 2 weeks. At Day 15, rats were euthanized, and brains removed. Brains were dissected into frontal cortex, hippocampus, cerebellum, and brainstem. Western blotting was performed to measure levels of total-Tau, phosphorylated-Tau (pTau), amyloid precursor protein (APP), GFAP, Iba1, αII-spectrin, and spectrin breakdown products (SBDP). Kinases and phosphatases, correlated with tau phosphorylation were also measured. Immunohistochemistry for pTau, APP, GFAP, and Iba1 was performed. pTau protein level was greater in the hippocampus, cerebellum, and brainstem and APP protein level was greater in cerebellum of blast vs control rats (p < 0.05). GFAP, Iba1, αII-spectrin, and SBDP remained unchanged. No immunohistochemical or neurobehavioral changes were observed. The dissociation between increased pTau and APP in different regions in the absence of neurobehavioral changes 2 weeks after double blast exposure is a relevant finding, consistent with human data showing that battlefield blasts might be associated with molecular changes before signs of neurological and psychiatric disorders manifest.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

