A site-specific map of the human plasma glycome and its age and gender-associated alterations
- PMID: 33060657
- PMCID: PMC7567094
- DOI: 10.1038/s41598-020-73588-x
A site-specific map of the human plasma glycome and its age and gender-associated alterations
Abstract
Alterations in the human glycome have been associated with cancer and autoimmunity. Thus, constructing a site-specific map of the human glycome for biomarker research and discovery has been a highly sought-after objective. However, due to analytical barriers, comprehensive site-specific glycoprofiling is difficult to perform. To develop a platform to detect easily quantifiable, site-specific, disease-associated glycan alterations for clinical applications, we have adapted the multiple reaction monitoring mass spectrometry method for use in glycan biomarker research. The adaptations allow for highly precise site-specific glycan monitoring with minimum sample prep. Using this technique, we successfully mapped out the relative abundances of the most common 159 glycopeptides in the plasma of 97 healthy volunteers. This plasma glycome map revealed 796 significant (FDR < 0.05) site-specific inter-protein and intra-protein glycan associations, of which the vast majority were previously unknown. Since age and gender are relevant covariants in biomarker research, these variables were also characterized. 13 glycopeptides were found to be associated with gender and 41 to be associated with age. Using just five age-associated glycopeptides, a highly accurate age prediction model was constructed and validated (r2 = 0.62 ± 0.12). The human plasma site-specific glycan map described herein has utility in applications ranging from glycan biomarker research and discovery to the development of novel glycan-altering interventions.
Conflict of interest statement
The authors declare no competing interests.
Figures


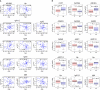
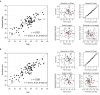
References
-
- National Research Council (US) Committee on Assessing the Importance and Impact of Glycomics and Glycosciences. Transforming Glycoscience: A Roadmap for the Future (National Academies Press, US, 2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

