Unique immunological profile in patients with COVID-19
- PMID: 33060840
- PMCID: PMC7557230
- DOI: 10.1038/s41423-020-00557-9
Unique immunological profile in patients with COVID-19
Abstract
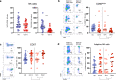
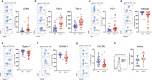
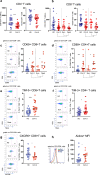
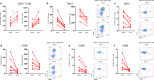
The relationship between severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and host immunity is poorly understood. We performed an extensive analysis of immune responses in 32 patients with severe COVID-19, some of whom succumbed. A control population of healthy subjects was included. Patients with COVID-19 had an altered distribution of peripheral blood lymphocytes, with an increased proportion of mature natural killer (NK) cells and low T-cell numbers. NK cells and CD8+ T cells overexpressed T-cell immunoglobulin and mucin domain-3 (TIM-3) and CD69. NK cell exhaustion was attested by increased frequencies of programmed cell death protein 1 (PD-1) positive cells and reduced frequencies of natural killer group 2 member D (NKG2D)-, DNAX accessory molecule-1 (DNAM-1)- and sialic acid-binding Ig-like lectin 7 (Siglec-7)-expressing NK cells, associated with a reduced ability to secrete interferon (IFN)γ. Patients with poor outcome showed a contraction of immature CD56bright and an expansion of mature CD57+ FcεRIγneg adaptive NK cells compared to survivors. Increased serum levels of IL-6 were also more frequently identified in deceased patients compared to survivors. Of note, monocytes secreted abundant quantities of IL-6, IL-8, and IL-1β which persisted at lower levels several weeks after recovery with concomitant normalization of CD69, PD-1 and TIM-3 expression and restoration of CD8+ T cell numbers. A hyperactivated/exhausted immune response dominate in severe SARS-CoV-2 infection, probably driven by an uncontrolled secretion of inflammatory cytokines by monocytes. These findings unveil a unique immunological profile in COVID-19 patients that will help to design effective stage-specific treatments for this potentially deadly disease.
Keywords: COVID-19; IL6; Monocytes; NK cells; TIM-3.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

