COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup
- PMID: 33060844
- PMCID: PMC7561246
- DOI: 10.1038/s41581-020-00356-5
COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup
Erratum in
-
Publisher Correction: COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup.Nat Rev Nephrol. 2020 Dec;16(12):765. doi: 10.1038/s41581-020-00372-5. Nat Rev Nephrol. 2020. PMID: 33139940 Free PMC article.
Abstract
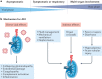
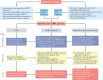
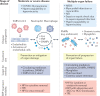
Kidney involvement in patients with coronavirus disease 2019 (COVID-19) is common, and can range from the presence of proteinuria and haematuria to acute kidney injury (AKI) requiring renal replacement therapy (RRT; also known as kidney replacement therapy). COVID-19-associated AKI (COVID-19 AKI) is associated with high mortality and serves as an independent risk factor for all-cause in-hospital death in patients with COVID-19. The pathophysiology and mechanisms of AKI in patients with COVID-19 have not been fully elucidated and seem to be multifactorial, in keeping with the pathophysiology of AKI in other patients who are critically ill. Little is known about the prevention and management of COVID-19 AKI. The emergence of regional 'surges' in COVID-19 cases can limit hospital resources, including dialysis availability and supplies; thus, careful daily assessment of available resources is needed. In this Consensus Statement, the Acute Disease Quality Initiative provides recommendations for the diagnosis, prevention and management of COVID-19 AKI based on current literature. We also make recommendations for areas of future research, which are aimed at improving understanding of the underlying processes and improving outcomes for patients with COVID-19 AKI.
Conflict of interest statement
L.G.F. has received grant and/or research support from Baxter and speaker’s honoraria from bioMérieux and Ortho Clinical diagnostics. R.L.M. has received grant and/or research support from Fresenius and Fresenius-Kabi and consulting fees from AM-Pharma, Sphingotec, Akebia, GE healthcare, Indalo, bioMérieux, Intercept, Baxter, Medtronic and Mallinckrodt. M.J.C. has received grant and/or research support from NIH and Potrero Medical Inc. K.D.L. has received consulting fees from bioMérieux and Durect. M.O. has received grant and/or research support from Baxter. T. Rimmelé has received grant and/or research support from Baxter and consulting fees from Baxter, Fresenius Medical Care, bioMérieux, Braun and Nikkiso. A.Z. has received grant and/or research support from Astute Medical, Fresenius, Baxter, Astellas, DFG, EK and consulting fees from Baxter, Astute Medical, bioMérieux, La Jolla Pharmaceuticals, Fresenius, Braun, AM-Pharma, Astellas and Radiopharma. S.L.G. has grant and/or research support and consulting fees from ExThera, BioPorto, Baxter and SeaStar, speaker’s honoraria from Baxter and Fresenius and is a stockholder for MediBeacon. S.G. has received consulting fees from GlaxoSmithKline. M.J. has received grant and/or research support from Baxter. J.L.K. has received grant and/or research support from NxStage, Satellite Health Care, and consulting fees from Astute Medical, Baxter, Sphingotec, and honoraria from the American Society of Nephrology. M.L. has received grant and/or research support from Sphingotec and consulting fees from Novartis. S.M. has received consulting fees from Angion Biomedica. X.L.P.-F. has received speaker’s honoraria from Baxter. P.P. has received travel and consulting fees from AM-Pharma, EBI and Sphingotec. J.P. has received grant and/or research support from bioMérieux, consulting fees from Quark Pharmaceutical and Medibeacon Inc., and speaker’s honoraria from Nikkiso Europe GmbH, Baxter, Braun Medical Ltd, Fresenius Medical Care and Fresenius-Kabi UK. T. Reis. has received consulting fees from Baxter and Eurofarma and speaker’s honoraria from Baxter and Braun. N.S. has received grant and/or research support from Baxter. A.T. has received consulting fees and speaker’s honoraria from Baxter and has a patent on 0.5% citrate solution for continuous renal replacement therapy. A.V. has received consulting fees for NxStage and has received other fees from Boehringer Ingelheim. G.V. has received grant and/or research support from Baxter SpA. C.R. has received consulting fees from Baxter, Jfron, bioMérieux, Medtronic and speaker’s honoraria from GE, OCD, and Cytosorbents. J.A.K. has received grant and/or research support from Astellas, Astute Medical, Baxter, bioMérieux, Cytosorbents, RenalSense, consulting fees from Astellas, Astute Medical, Baxter, bioMérieux, Cytosorbents, RenalSense, DaVita, Fresenius, Mallinckrodt, NxStage, Potrero, and has licensing of intellectual property for Astute Medical and Cytosorbents. The other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

