Saliva as an Alternative Specimen for Molecular COVID-19 Testing in Community Settings and Population-Based Screening
- PMID: 33061486
- PMCID: PMC7534854
- DOI: 10.2147/IDR.S275152
Saliva as an Alternative Specimen for Molecular COVID-19 Testing in Community Settings and Population-Based Screening
Abstract
Purpose: With the easing of restriction measures, repeated community-based sampling for tracking new COVID-19 infections is anticipated for the next 6 to 12 months. A non-invasive, self-collected specimen like saliva will be useful for such public health surveillance. Investigations on the use of saliva for SARS-CoV-2 RT-PCR have largely been among COVID-19 in-pa\tients and symptomatic ambulatory patients with limited work in a community-based screening setting. This study was carried out to address this paucity of data and reported discrepancies in diagnostic accuracy for saliva samples.
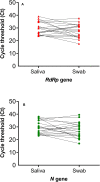
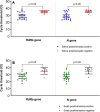
Patients and methods: From 29th June to 14th July 2020, adults presenting for COVID-19 testing at a community-based screening facility in Dubai, United Arab Emirates were recruited. Clinical data, nasopharyngeal swab in universal transport media and drooling saliva in sterile containers were obtained. Reverse transcriptase PCR amplification of SARS-CoV-2 RdRp and N genes was used to detect the presence of the SARS-CoV-2 virus.
Results: Of the 401 participants, 35 (8.7%) had viral detection in at least one specimen type and the majority (n=20/35; 57.1%) were asymptomatic. Both swab and saliva were positive in 19 (54.2%) patients, while 7 (20.0%) patients had swab positive/saliva negative results. There were 9 (25.7%) patients with saliva positive/swab negative result and this included 5 asymptomatic COVID-19 patients undergoing repeat screening. Using the swab as the reference gold standard, the sensitivity and specificity of saliva were 73.1% (95% CI 52.2-88.4%) and 97.6% (95% CI 95.5-98.9%) while the positive and negative predictive values were 67.9% (95% CI 51.5-80.8%) and 98.1% (95% CI 96.5-99.0%), respectively.
Conclusion: The findings suggest good diagnostic accuracy for saliva and feasibility of utilization of specimen without transport media for SARS-CoV-2 RT-PCR. Saliva represents a potential specimen of choice in community settings and population-based screening.
Keywords: SARS-CoV-2; molecular test; nasopharyngeal swab; population-based screening; saliva.
© 2020 Senok et al.
Conflict of interest statement
YA, JAZ are employees of Unilabs UAE but this did not influence the study design, and they have no competing interests to declare. None of the other authors has any financial or other relationships that may constitute a conflict of interest.
Figures
Similar articles
-
The diagnostic accuracy of RT-PCR from self-collected saliva versus nasopharyngeal sampling: A systematic review and meta-analysis.Saudi Med J. 2022 Jan;43(1):9-30. doi: 10.15537/smj.2022.43.1.20210743. Saudi Med J. 2022. PMID: 35022280 Free PMC article.
-
Saliva for molecular detection of SARS-CoV-2 in school-age children.Clin Microbiol Infect. 2021 Sep;27(9):1330-1335. doi: 10.1016/j.cmi.2021.02.009. Epub 2021 Feb 19. Clin Microbiol Infect. 2021. PMID: 33618013 Free PMC article.
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Comparison of SARS-CoV-2 PCR-Based Detection Using Saliva or Nasopharyngeal Swab Specimens in Asymptomatic Populations.Microbiol Spectr. 2021 Sep 3;9(1):e0006221. doi: 10.1128/Spectrum.00062-21. Epub 2021 Aug 25. Microbiol Spectr. 2021. PMID: 34431689 Free PMC article.
-
Salivary SARS-CoV-2 RNA for diagnosis of COVID-19 patients: a systematic revisew and meta-analysis of diagnostic accuracy.Jpn Dent Sci Rev. 2023 Jun 21;59:219-38. doi: 10.1016/j.jdsr.2023.06.004. Online ahead of print. Jpn Dent Sci Rev. 2023. PMID: 37360001 Free PMC article. Review.
Cited by
-
SARS-CoV-2 antigen detection by saliva; an alternative to nasopharyngeal specimen: A cross-sectional study.Health Sci Rep. 2023 May 18;6(5):e1275. doi: 10.1002/hsr2.1275. eCollection 2023 May. Health Sci Rep. 2023. PMID: 37216057 Free PMC article.
-
One-Year Update on Salivary Diagnostic of COVID-19.Front Public Health. 2021 May 21;9:589564. doi: 10.3389/fpubh.2021.589564. eCollection 2021. Front Public Health. 2021. PMID: 34150692 Free PMC article. Review.
-
RT-qPCR-based tests for SARS-CoV-2 detection in pooled saliva samples for massive population screening to monitor epidemics.Sci Rep. 2022 May 16;12(1):8082. doi: 10.1038/s41598-022-12179-4. Sci Rep. 2022. PMID: 35577836 Free PMC article.
-
The diagnostic accuracy of RT-PCR from self-collected saliva versus nasopharyngeal sampling: A systematic review and meta-analysis.Saudi Med J. 2022 Jan;43(1):9-30. doi: 10.15537/smj.2022.43.1.20210743. Saudi Med J. 2022. PMID: 35022280 Free PMC article.
-
Screening for SARS-CoV-2 by RT-PCR: Saliva or nasopharyngeal swab? Rapid review and meta-analysis.PLoS One. 2021 Jun 10;16(6):e0253007. doi: 10.1371/journal.pone.0253007. eCollection 2021. PLoS One. 2021. PMID: 34111196 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

