Management of Immune Checkpoint Inhibitor Toxicities
- PMID: 33061607
- PMCID: PMC7533913
- DOI: 10.2147/CMAR.S218756
Management of Immune Checkpoint Inhibitor Toxicities
Abstract
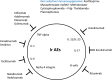
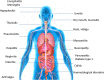
Immune checkpoint inhibitors (ICIs) have radically changed the clinical outcome of several cancers with durable responses. CTLA-4 (cytotoxic T lymphocyte antigen-4), PD-1 (programmed cell death protein 1) or PDL-1 (programmed cell death ligand protein 1) represent ICIs that can be used as monotherapy or in combination with other agents. The toxicity p\rofiles of ICIs differ from the side effects of cytotoxic agents and come with new toxicities like immune-related adverse events. Typically, these toxicities occur in all organs. However, the main organs affected are the skin, digestive, hepatic, lungs, rheumatologic, and endocrine. Most of the immune toxicity that occurs is low grade but some more severe toxicities can occur that require a rapid diagnosis and appropriate treatment. The recognition of symptoms by physicians and patient is necessary to resolve them rapidly and adapt treatment to allow the toxicity to resolve.
Keywords: corticosteroids; immune check point inhibitor; immunosuppressive treatments; toxicity.
© 2020 Durrechou et al.
Conflict of interest statement
Felix Lefort reports personal fees from Ipsen, outside the submitted work. A Ravaud has received honoraria for consultancy from Pfizer, Astra Zeneca, MSD, BMS, Roche, and Ipsen; is a member of the GU for Pfizer, Astra Zeneca, MSD, BMS, Roche and Ipsen; and has received transportation and housing for meetings from Pfizer, Astra Zeneca, BMS and Ipsen; and reports grants, personal fees, and non-financial support from Pfizer and Merck GA, and personal fees, non-financial support from BMS, Ipsen, MSD, Astra Zeneca, and Novartis, outside the submitted work. M Gross-Goupil is a member of GU board for Pfizer, Astra Zeneca, MSD, BMS, Roche and Ipsen; has received transportation and housing for meetings from Pfizer, BMS, Janssen, Ipsen, Roche, MSD, Amgen, Astellas; has received honoraria for symposiums from MSD, BMS, Pfizer.; and reports personal fees, non-financial support from BMS, MSD, Astra Zeneca, Roche, and Merck, during the conduct of the study. A Daste has received honoraria for consultancy for Astra-Zeneca, BS, MSD, Roche, and Merck. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

