CircCFL1/MiR-107 Axis Targeting HMGB1 Promotes the Malignant Progression of Diffuse Large B-Cell Lymphoma Tumors
- PMID: 33061624
- PMCID: PMC7533230
- DOI: 10.2147/CMAR.S263222
CircCFL1/MiR-107 Axis Targeting HMGB1 Promotes the Malignant Progression of Diffuse Large B-Cell Lymphoma Tumors
Abstract
Objective: The pathogenesis of diffuse large B-cell lymphoma (DLBCL) has not yet been fully elucidated. An increasing number of studies have shown that circular RNAs (circRNAs) play an important role in tumorigenesis and development. The aim of this study was to investigate the effect of CircCFL1 on the malignant progression of DLBCL.
Methods: RT-qPCR was used to detect the expression levels of CircCFL1 and miR-107. A dual-luciferase reporter gene experiment was conducted to verify that CircCFL1 targeted miR-107 and the miR-107 target gene HMGB1. BrdU, transwell, and MTT tests were performed to detect cell invasion and proliferation. Western blot analysis was used to detect the phosphorylation of proteins. Xenograft models were established to confirm the effect of CircCFL1 on DLBCL tumor growth in vivo.
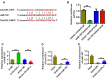
Results: The expression of CircCFL1 in cells transfected with the CircCFL1 overexpression vector was higher than that in the control group. After overexpressing CircCFL1, the expression of miR-107 in cells decreased significantly, and the protein level of HMGB1 increased. The dual-luciferase reporter gene experiment showed that CircCFL1 directly bound to miR-107 and reduced the inhibition of the target gene HMGB1. After CircCFL1 was overexpressed, cell migration and proliferation were enhanced. The tumor volume and weight in the lentivirus CircCFL1 group were higher than those in the lentivirus NC group.
Conclusion: Results showed that the circRNA CircCFL1 could regulate the expression of HMGB1 through miR-107 to promote the proliferation and migration of DLBCL.
Keywords: CircCFL1; HMGB1; circular RNA; lymphoma; migration; proliferation.
© 2020 Chen et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






References
-
- Ludmir EB, Milgrom SA, Pinnix CC, Gunther JR, Nastoupil LJJLL. Primary breast diffuse large B-cell lymphoma: treatment strategies and patterns of failure. Leuk Lymphoma. 2018;59(12):2896–2903. - PubMed
LinkOut - more resources
Full Text Sources

