Alcohol Use Disorder, Neurodegeneration, Alzheimer's and Parkinson's Disease: Interplay Between Oxidative Stress, Neuroimmune Response and Excitotoxicity
- PMID: 33061892
- PMCID: PMC7488355
- DOI: 10.3389/fncel.2020.00282
Alcohol Use Disorder, Neurodegeneration, Alzheimer's and Parkinson's Disease: Interplay Between Oxidative Stress, Neuroimmune Response and Excitotoxicity
Abstract
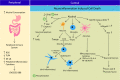
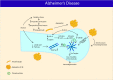
Alcohol use disorder (AUD) has been associated with neurodegenerative diseases such as Alzheimer's and Parkinson's disease. Prolonged excessive alcohol intake contributes to increased production of reactive oxygen species that triggers neuroimmune response and cellular apoptosis and necrosis via lipid peroxidation, mitochondrial, protein or DNA damage. Long term binge alcohol consumption also upregulates glutamate receptors, glucocorticoids and reduces reuptake of glutamate in the central nervous system, resulting in glutamate excitotoxicity, and eventually mitochondrial injury and cell death. In this review, we delineate the following principles in alcohol-induced neurodegeneration: (1) alcohol-induced oxidative stress, (2) neuroimmune response toward increased oxidants and lipopolysaccharide, (3) glutamate excitotoxicity and cell injury, and (4) interplay between oxidative stress, neuroimmune response and excitotoxicity leading to neurodegeneration and (5) potential chronic alcohol intake-induced development of neurodegenerative diseases, including Alzheimer's and Parkinson's disease.
Keywords: Alzheimer; Parkinson; alcohol; excitotoxicity; neurodegeneration; neuroimmune; neuroinflammation; oxidative.
Copyright © 2020 Kamal, Tan, Ibrahim, Shaikh, Mohamed, Mohamed, Hamid, Ugusman and Kumar.
Figures


References
-
- Ambhore N. S., Mali J. K., Kanhed A. M., Antony S., Bhalerao A. R., Bhojraj S. (2012). Pharmacological and biochemical interventions of cigarette smoke, alcohol, and sexual mating frequency on idiopathic rat model of Parkinson’s disease. J. Young Pharm. 4 177–183. 10.4103/0975-1483.100026 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

