Virtual Screening Technique Used to Estimate the Mechanism of Adhatoda vasica Nees for the Treatment of Rheumatoid Arthritis Based on Network Pharmacology and Molecular Docking
- PMID: 33062015
- PMCID: PMC7542480
- DOI: 10.1155/2020/5872980
Virtual Screening Technique Used to Estimate the Mechanism of Adhatoda vasica Nees for the Treatment of Rheumatoid Arthritis Based on Network Pharmacology and Molecular Docking
Abstract
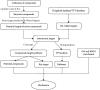
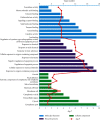
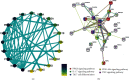
Adhatoda vasica Nees (AVN) is commonly used to treat joint diseases such as rheumatoid arthritis (RA) in ethnic minority areas of China, especially in Tibetan and Dai areas, and its molecular mechanisms on RA still remain unclear. Network pharmacology, a novel strategy, utilizes bioinformatics to predict and evaluate drug targets and interactions in disease. Here, network pharmacology was used to investigate the mechanism by which AVN acts in RA. The chemical compositions and functional targets of AVN were retrieved using the systematic pharmacological analysis platform PharmMapper. The targets of RA were queried through the DrugBank database. The protein-protein interaction network (PPI), Gene Ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses of key targets were constructed in the STRING database, and the network visualization analysis was performed in Cytoscape. Maestro 11.1, a type of professional software, was used for verifying prediction and analysis based on network pharmacology. By comparing the predicted target information with the targets of RA-related drugs, 25 potential targets may be related to the treatment of RA, among which MAPK1, TNF, DHODH, IL2, PTGS2, and JAK2 may be the main potential targets for the treatment of RA. Finally, the chemical components and potential target proteins were scored by molecular docking, and compared with the ligands of the protein, the prediction results of network pharmacology were preliminarily verified. The active ingredients and mechanism of AVN against RA were firstly investigated using network pharmacology. Additionally, this research provided a solid foundation for further experimental studies.
Copyright © 2020 Wenxiang Wang et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures




Similar articles
-
[Anti-rheumatoid arthritis mechanism of Sophorae Tonkinesis Radix et Rhizoma based on network pharmacology and experimental verification].Zhongguo Zhong Yao Za Zhi. 2022 Oct;47(19):5327-5335. doi: 10.19540/j.cnki.cjcmm.20220526.401. Zhongguo Zhong Yao Za Zhi. 2022. PMID: 36472040 Chinese.
-
[Mechanism of Chaenomelis Fructus in treatment of rheumatoid arthritis based on network pharmacology and experimental verification].Zhongguo Zhong Yao Za Zhi. 2023 Sep;48(18):4852-4863. doi: 10.19540/j.cnki.cjcmm.20221212.402. Zhongguo Zhong Yao Za Zhi. 2023. PMID: 37802827 Chinese.
-
[Mechanism of Olibanum-Myrrha in treatment of rheumatoid arthritis based on network pharmacology and molecular docking].Zhongguo Zhong Yao Za Zhi. 2021 May;46(10):2371-2379. doi: 10.19540/j.cnki.cjcmm.20201126.401. Zhongguo Zhong Yao Za Zhi. 2021. PMID: 34047081 Chinese.
-
Exploring the pharmacological components and effective mechanism of Mori Folium against periodontitis using network pharmacology and molecular docking.Arch Oral Biol. 2022 Jul;139:105391. doi: 10.1016/j.archoralbio.2022.105391. Epub 2022 Mar 21. Arch Oral Biol. 2022. PMID: 35430443
-
Exploration of the mechanism of Zisheng Shenqi decoction against gout arthritis using network pharmacology.Comput Biol Chem. 2021 Feb;90:107358. doi: 10.1016/j.compbiolchem.2020.107358. Epub 2020 Aug 8. Comput Biol Chem. 2021. PMID: 33243703 Review.
Cited by
-
An Investigation of the Molecular Mechanisms Underlying the Analgesic Effect of Jakyak-Gamcho Decoction: A Network Pharmacology Study.Evid Based Complement Alternat Med. 2020 Dec 1;2020:6628641. doi: 10.1155/2020/6628641. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33343676 Free PMC article.
-
A study of the therapeutic mechanism of Jakyakgamcho-Tang about functional dyspepsia through network pharmacology research.Int J Med Sci. 2022 Oct 17;19(13):1824-1834. doi: 10.7150/ijms.77451. eCollection 2022. Int J Med Sci. 2022. PMID: 36438925 Free PMC article.
-
Elucidating the material basis and potential mechanisms of Ershiwuwei Lvxue Pill acting on rheumatoid arthritis by UPLC-Q-TOF/MS and network pharmacology.PLoS One. 2022 Feb 7;17(2):e0262469. doi: 10.1371/journal.pone.0262469. eCollection 2022. PLoS One. 2022. PMID: 35130279 Free PMC article.
-
Long Non-Coding RNA NR-133666 Promotes the Proliferation and Migration of Fibroblast-Like Synoviocytes Through Regulating the miR-133c/MAPK1 Axis.Front Pharmacol. 2022 Apr 1;13:887330. doi: 10.3389/fphar.2022.887330. eCollection 2022. Front Pharmacol. 2022. PMID: 35431959 Free PMC article.
-
Uncovering the molecular mechanism of Gynostemma pentaphyllum (Thunb.) Makino against breast cancer using network pharmacology and molecular docking.Medicine (Baltimore). 2022 Dec 9;101(49):e32165. doi: 10.1097/MD.0000000000032165. Medicine (Baltimore). 2022. PMID: 36626523 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

