Hydrological and soil physiochemical variables determine the rhizospheric microbiota in subtropical lakeshore areas
- PMID: 33062450
- PMCID: PMC7531358
- DOI: 10.7717/peerj.10078
Hydrological and soil physiochemical variables determine the rhizospheric microbiota in subtropical lakeshore areas
Abstract
Background: Due to intensive sluice construction and other human disturbances, lakeshore vegetation has been destroyed and ecosystems greatly changed. Rhizospheric microbiota constitute a key part of a functioning rhizosphere ecosystem. Maintaining rhizosphere microbial diversity is a central, critical issue for sustaining these rhizospheric microbiota functions and associated ecosystem services. However, the community composition and abiotic factors influencing rhizospheric microbiota in lakeshore remain largely understudied.
Methods: The spatiotemporal composition of lakeshore rhizospheric microbiota and the factors shaping them were seasonally investigated in three subtropical floodplain lakes (Lake Chaohu, Lake Wuchang, and Lake Dahuchi) along the Yangtze River in China through 16S rRNA amplicon high-throughput sequencing.
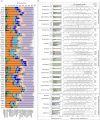
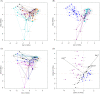
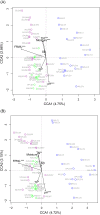
Results: Our results showed that four archaeal and 21 bacterial phyla (97.04 ± 0.25% of total sequences) dominated the rhizospheric microbiota communities of three lakeshore areas. Moreover, we uncovered significant differences among rhizospheric microbiota among the lakes, seasons, and average submerged depths. The Acidobacteria, Actinobacteria, Bacteroidetes, Bathyarchaeota, Gemmatimonadetes, and Proteobacteria differed significantly among the three lakes, with more than half of these dominant phyla showing significant changes in abundance between seasons, while the DHVEG-6, Ignavibacteriae, Nitrospirae, Spirochaetes, and Zixibacteria varied considerably across the average submerged depths (n = 58 sites in total). Canonical correspondence analyses revealed that the fluctuation range of water level and pH were the most important factors influencing the microbial communities and their dominant microbiota, followed by total nitrogen, moisture, and total phosphorus in soil. These results suggest a suite of hydrological and soil physiochemical variables together governed the differential structuring of rhizospheric microbiota composition among different lakes, seasons, and sampling sites. This work thus provides valuable ecological information to better manage rhizospheric microbiota and protect the vegetation of subtropical lakeshore areas.
Keywords: Hydrology; Lakeshore area; Microbial community; Rhizospheric microbiota.
©2020 Zhang et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures



References
-
- Baastrup-Spohr L, Sand-Jensen K, Olesen SCH, Bruun HH. Recovery of lake vegetation following reduced eutrophication and acidification. Freshwater Biology. 2017;62:1847–1857. doi: 10.1111/fwb.13000. - DOI
-
- Bååth E, Anderson TH. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biology and Biochemistry. 2003;35:955–963. doi: 10.1016/S0038-0717(03)00154-8. - DOI
-
- Bayley S, Guimond JK. Effects of river connectivity on marsh vegetation community structure and species richness in Montana floodplain wetlands in Jasper National Park, Alberta, Canada. Ecoscience. 2008;15:377–388. doi: 10.2980/15-3-3084. - DOI
LinkOut - more resources
Full Text Sources

