Impact of Patient Characteristics on Treatment Outcomes in Symptomatic Venous Thromboembolism: Results of HOKUSAI-VTE Randomized Trial Analysis
- PMID: 33062927
- PMCID: PMC7553796
- DOI: 10.1055/s-0040-1716496
Impact of Patient Characteristics on Treatment Outcomes in Symptomatic Venous Thromboembolism: Results of HOKUSAI-VTE Randomized Trial Analysis
Abstract
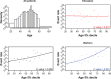
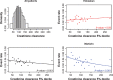
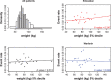
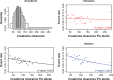
Introduction In patients with venous thromboembolism (VTE), direct oral anticoagulants (DOACs) such as edoxaban, apixaban, dabigatran, and rivaroxaban are more convenient, safer, and just as effective as vitamin K antagonists (VKAs). Limited information is known about the effects of patient characteristics on VTE efficacy and safety of DOACs compared with VKAs, without appropriate effect modifier adjustment comparisons of DOACs may be biased. This study considers the effect of variables that can modify the efficacy and safety of edoxaban and warfarin, using patient-level data. Materials and Methods The primary efficacy and safety outcomes in the HOKUSAI-VTE study were VTE recurrence and clinically relevant bleeding, respectively. Potential effect modifiers were age, creatinine clearance, and weight. The relationship between the percentage of time in international normalized ratio (INR) control and outcomes were considered for the warfarin arm. Univariate and multivariate regression were performed for each patient characteristic. Results The relationship between treatment and VTE recurrence differed by age (interaction p = 0.007) and by creatinine clearance ( p = 0.05). VTE recurrence differed by age for patients in the warfarin arm but not for those in the edoxaban arm and differed by INR control in the warfarin arm ( p < 0.005). A stronger relationship between creatinine clearance and clinically relevant bleeding was found in the warfarin arm than in the edoxaban arm ( p = 0.04). Clinically relevant bleeding differed by the percentage of time in INR control in the warfarin arm ( p < 0.005). Age appeared to be a more important effect modifier than creatinine clearance in patients with VTE. Discussion The finding that efficacy in older patients was greater for those taking edoxaban than for those taking warfarin in the HOKUSAI-VTE study needs further investigation. Modification of the treatment effect by age for those taking warfarin might bias estimates of comparative effectiveness among DOACs if VKAs are the reference treatment.
Keywords: age; creatinine; edoxaban; effect modification; venous thrombosis; warfarin.
Conflict of interest statement
Conflict of Interest B.v.H. has served as a consultant for Daiichi-Sankyo. E.H. has served as a consultant for Daiichi-Sankyo. A.T.C. has served as a consultant and received honoraria from Daiichi-Sankyo for participating in the PREFER registry advisory committee; he also receives consultancy and honoraria from other pharmaceutical companies such as Bayer, Pfizer, BMS, Portola, and Aspen.
Figures


References
-
- Martinez C, Cohen A T, Bamber L, Rietbrock S. Epidemiology of first and recurrent venous thromboembolism: a population-based cohort study in patients without active cancer. Thromb Haemost. 2014;112(02):255–263. - PubMed
-
- VTE Impact Assessment Group in Europe (VITAE) . Cohen A T, Agnelli G, Anderson F A. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(04):756–764. - PubMed
-
- AMPLIFY Investigators . Agnelli G, Buller H R, Cohen A. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(09):799–808. - PubMed
-
- EINSTEIN Investigators . Bauersachs R, Berkowitz S D, Brenner B. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. - PubMed

