Increased epithelial membrane protein 2 expression in glioblastoma after treatment with bevacizumab
- PMID: 33063013
- PMCID: PMC7542982
- DOI: 10.1093/noajnl/vdaa112
Increased epithelial membrane protein 2 expression in glioblastoma after treatment with bevacizumab
Abstract
Background: Antiangiogenic therapy with bevacizumab has failed to provide substantial gains in overall survival. Epithelial membrane protein 2 (EMP2) is a cell surface protein that has been previously shown to be expressed in glioblastoma, correlate with poor survival, and regulate neoangiogenesis in cell lines. Thus, the relationship between bevacizumab and EMP2 was investigated.
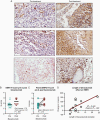
Methods: Tumor samples were obtained from 12 patients with newly diagnosed glioblastoma at 2 time points: (1) during the initial surgery and (2) during a subsequent surgery following disease recurrence post-bevacizumab treatment. Clinical characteristics and survival data from these patients were collected, and tumor samples were stained for EMP2 expression. The IVY Glioblastoma Atlas Project database was used to evaluate EMP2 expression levels in 270 samples by differing histological areas of the tumor.
Results: Patients with high EMP2 staining at initial diagnosis had decreased progression-free and overall survival after bevacizumab (median progression-free survival 4.6 months vs 5.9 months; log-rank P = .076 and overall survival 7.7 months vs 14.4 months; log-rank P = .011). There was increased EMP2 staining in samples obtained after bevacizumab treatment in both unpaired (mean H-score 2.31 vs 1.76; P = .006) and paired analyses (mean difference 0.571; P = .019). This expression increase correlated with length of bevacizumab therapy (R 2 = 0.449; Pearson P = .024).
Conclusions: Bevacizumab treatment increased EMP2 protein expression. This increase in EMP2 correlated with reduced mean survival time post-bevacizumab therapy. We hypothesize a role of EMP2 in clinical bevacizumab resistance and as a potential antiangiogenic therapeutic target in glioblastoma.
Keywords: EMP2; angiogenesis; bevacizumab; glioblastoma.
© The Author(s) 2020. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Figures

Similar articles
-
Epithelial membrane protein-2 (EMP2) promotes angiogenesis in glioblastoma multiforme.J Neurooncol. 2017 Aug;134(1):29-40. doi: 10.1007/s11060-017-2507-8. Epub 2017 Jun 9. J Neurooncol. 2017. PMID: 28597184 Free PMC article.
-
Tissue microarray analysis for epithelial membrane protein-2 as a novel biomarker for gliomas.Brain Tumor Pathol. 2018 Jan;35(1):1-9. doi: 10.1007/s10014-017-0300-1. Epub 2017 Sep 8. Brain Tumor Pathol. 2018. PMID: 28887715 Free PMC article.
-
Epithelial membrane protein-2 (EMP2) activates Src protein and is a novel therapeutic target for glioblastoma.J Biol Chem. 2014 May 16;289(20):13974-85. doi: 10.1074/jbc.M113.543728. Epub 2014 Mar 18. J Biol Chem. 2014. PMID: 24644285 Free PMC article.
-
Progression-Free but No Overall Survival Benefit for Adult Patients with Bevacizumab Therapy for the Treatment of Newly Diagnosed Glioblastoma: A Systematic Review and Meta-Analysis.Cancers (Basel). 2019 Nov 4;11(11):1723. doi: 10.3390/cancers11111723. Cancers (Basel). 2019. PMID: 31689995 Free PMC article. Review.
-
Epithelial membrane protein 2: Molecular interactions and clinical implications.J Clin Neurosci. 2017 Oct;44:84-88. doi: 10.1016/j.jocn.2017.06.044. Epub 2017 Jul 15. J Clin Neurosci. 2017. PMID: 28720310 Review.
Cited by
-
A-to-I RNA editing shows dramatic up-regulation in osteosarcoma and broadly regulates tumor-related genes by altering microRNA target regions.J Appl Genet. 2023 Sep;64(3):493-505. doi: 10.1007/s13353-023-00777-5. Epub 2023 Aug 5. J Appl Genet. 2023. PMID: 37542613
-
Systematic Review of Molecular Targeted Therapies for Adult-Type Diffuse Glioma: An Analysis of Clinical and Laboratory Studies.Int J Mol Sci. 2023 Jun 21;24(13):10456. doi: 10.3390/ijms241310456. Int J Mol Sci. 2023. PMID: 37445633 Free PMC article.
-
The Multifaceted Role of Epithelial Membrane Protein 2 in Cancer: from Biomarker to Therapeutic Target.Biomol Ther (Seoul). 2024 Nov 1;32(6):697-707. doi: 10.4062/biomolther.2024.168. Epub 2024 Oct 21. Biomol Ther (Seoul). 2024. PMID: 39428387 Free PMC article. Review.
-
Exploring the Past, Present, and Future of Anti-Angiogenic Therapy in Glioblastoma.Cancers (Basel). 2023 Jan 29;15(3):830. doi: 10.3390/cancers15030830. Cancers (Basel). 2023. PMID: 36765787 Free PMC article. Review.
References
-
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. - PubMed
-
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. - PubMed
-
- Sivakumar B, Harry LE, Paleolog EM. Modulating angiogenesis: more vs less. JAMA. 2004;292(8):972–977. - PubMed
