Physiologic Response to Angiotensin II Treatment for Coronavirus Disease 2019-Induced Vasodilatory Shock: A Retrospective Matched Cohort Study
- PMID: 33063034
- PMCID: PMC7523856
- DOI: 10.1097/CCE.0000000000000230
Physiologic Response to Angiotensin II Treatment for Coronavirus Disease 2019-Induced Vasodilatory Shock: A Retrospective Matched Cohort Study
Abstract
Objectives: To assess the early physiologic response to angiotensin-II treatment in patients with coronavirus disease 2019-induced respiratory failure and distributive shock.
Design: Retrospective consecutive-sample cohort study.
Setting: Three medical ICUs in New York during the coronavirus disease 2019 outbreak.
Patients: All patients were admitted to the ICU with respiratory failure and were receiving norepinephrine for distributive shock.
Interventions: The treatment groups were patients who received greater than or equal to 1 hour of angiotensin-II treatment. Time-zero was the time of angiotensin-II initiation. Controls were identified using a 2:1 hierarchical process that matched for 1) date and unit of admission; 2) specific organ support modalities; 3) age; 4) chronic lung, cardiovascular, and kidney disease; and 5) sex. Time-zero in the control group was 21 hours post vasopressor initiation, the mean duration of vasopressor therapy prior to angiotensin-II initiation in the treated group.
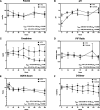
Measurements and main results: Main outcomes were trajectories of vasopressor requirements (in norepinephrine-equivalent dose) and mean arterial pressure. Additionally assessed trajectories were respiratory (Pao2/Fio2, Paco2), metabolic (pH, creatinine), and coagulation (d-dimer) dysfunction indices after time-zero. We also recorded adverse events and clinical outcomes. Trajectories were analyzed using mixed-effects models for immediate (first 6 hr), early (48 hr), and sustained (7 d) responses. Twenty-nine patients (n = 10 treated, n = 19 control) were identified. Despite matching, angiotensin-II-treated patients had markedly greater vasopressor requirements (mean: 0.489 vs 0.097 µg/kg/min), oxygenation impairment, and acidosis at time-zero. Nonetheless, angiotensin-II treatment was associated with an immediate and sustained reduction in norepinephrine-equivalent dose (6 hr model: β = -0.036 µg/kg/min/hr; 95% CI: -0.054 to -0.018 µg/kg/min/hr, p interaction=0.0002) (7 d model: β = -0.04 µg/kg/min/d, 95% CI: -0.05 to -0.03 µg/kg/min/d; p interaction = 0.0002). Compared with controls, angiotensin-II-treated patients had significantly faster improvement in mean arterial pressure, hypercapnia, acidosis, baseline-corrected creatinine, and d-dimer. Three thrombotic events occurred, all in control patients.
Conclusions: Angiotensin-II treatment for coronavirus disease 2019-induced distributive shock was associated with rapid improvement in multiple physiologic indices. Angiotensin-II in coronavirus disease 2019-induced shock warrants further study.
Keywords: angiotensin II; coronavirus disease 2019; norepinephrine; severe acute respiratory syndrome coronavirus-2 infection; shock; vasoconstrictor agents.
Copyright © 2020 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
Dr. Leisman discloses that La Jolla Pharmaceutical Company, the manufacturer of Giapreza, provided a sample of their drug free of charge for mouse experiments that are not related to the present study, and La Jolla Pharmaceutical Company had no access to any of that data or any role in its analysis, interpretation, or eventual dissemination and has had no role whatsoever in any aspect of the present study. Dr. Taylor discloses grant support from the National Institutes of Health (NIH). Dr. Deutschman discloses grant support from the NIH and consulting fees from Enlivex Therapeutics. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

