Impact of Food and Drink Administration Vehicles on Paediatric Formulation Performance Part 2: Dissolution of Montelukast Sodium and Mesalazine Formulations
- PMID: 33063245
- PMCID: PMC7561592
- DOI: 10.1208/s12249-020-01815-9
Impact of Food and Drink Administration Vehicles on Paediatric Formulation Performance Part 2: Dissolution of Montelukast Sodium and Mesalazine Formulations
Abstract
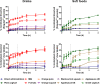
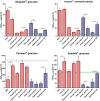
Paediatric medicines are not always age-appropriate, causing problems with dosing, acceptability and adherence. The use of food and drinks as vehicles for medicine co-administration is common practice, yet the impact on drug bioavailability, safety and efficacy remains unaddressed. The aim of this study was to use in vitro dissolution testing, under infant simulating conditions, to evaluate the effect of co-administration with vehicles on the dissolution performance of two poorly soluble paediatric drugs. Dissolution studies of mesalazine and montelukast formulations were conducted with mini-paddle apparatus on a two-stage approach: simulated gastric fluid followed by addition of simulated intestinal fluid. The testing scenarios were designed to reflect daily administration practices: direct administration of formulation; formulation co-administered with food and drinks, both immediately after mixing and 4 h after mixing. Drug dissolution was significantly affected by medicine co-administration with vehicles, compared to the direct administration of formulation. Furthermore, differences were observed on drug dissolution when the formulations were mixed with different vehicles of the same subtype. The time between preparation and testing of the drug-vehicle mixture also impacted dissolution behaviour. Drug dissolution was shown to be significantly affected by the physicochemical properties and composition of the vehicles, drug solubility in each vehicle and drug/formulation characteristics. Ultimately, in this study, we show the potential of age-appropriate in vitro dissolution testing as a useful biopharmaceutical tool for estimating drug dissolution in conditions relevant to the paediatric population. The setup developed has potential to evaluate the impact of medicine co-administration with vehicles on paediatric formulation performance.
Keywords: dissolution; drinks; drug manipulation; food; mini-paddle; multivariate analysis; paediatrics.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

