Neocortical injury-induced status epilepticus
- PMID: 33063874
- PMCID: PMC8764937
- DOI: 10.1111/epi.16715
Neocortical injury-induced status epilepticus
Abstract
Objective: To characterize neocortical onset status epilepticus (SE) in the C57BL/6J mouse.
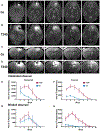
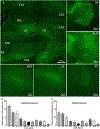
Methods: We induced SE by administering homocysteine 16-18 hours after cobalt (Co) implantation. SE was monitored by video and electroencephalography (EEG). We evaluated brain structure with magnetic resonance imaging (MRI). Neurodegeneration was evaluated 72 hours after SE using Fluoro-Jade C staining.
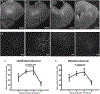
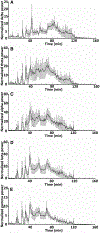
Results: Cobalt triggered seizures in a dose-dependent manner (median effective dose, ED50 = 0.78 mg) and the latency to peak seizure frequency shortened with increased dose. Animals developed SE after homocysteine administration. SE began with early intermittent focal seizures, consisting of frontal onset rhythmic spike-wave discharges manifested as focal dystonia with clonus. These focal seizures then evolved into generalized continuous convulsive activity. Behavioral manifestations of SE included tonic stiffening, bilateral limb clonus, and bilateral tonic-clonic movements, which were accompanied by generalized rhythmic spike-wave discharges on EEG. After prolonged seizures, animals became comatose with intermittent bilateral myoclonic seizures or jerks. During this period, EEG showed seizures interspersed with generalized periodic discharges on a suppressed background. MRI obtained when animals were in a coma revealed edema, midline shift in frontal lobe around the Co implantation site, and ventricular effacement. Fluoro-Jade C staining revealed neurodegeneration in the cortex, amygdala, and thalamus.
Significance: We have developed a mouse model of severe, refractory cortical-onset SE, consisting of convulsions merging into a coma, EEG patterns of cortical seizures, and injury, with evidence of widespread neocortical edema and damage. This model replicates many features of acute seizures and SE resulting from traumatic brain injury, subarachnoid, and lobar hemorrhage.
Keywords: Fluoro-Jade C; burst suppression; cobalt; edema; seizure.
© 2020 International League Against Epilepsy.
Conflict of interest statement
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
Figures


References
-
- Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus – report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56:1515–23. - PubMed
-
- DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–35. - PubMed
-
- Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia. 1994;35:27–34. - PubMed
-
- Neligan A, Shorvon SD. Frequency and prognosis of convulsive status epilepticus of different causes: a systematic review. Arch Neurol. 2010;67:931–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

