Rational Design of Azastatin as a Potential ADC Payload with Reduced Bystander Killing
- PMID: 33063934
- PMCID: PMC7756782
- DOI: 10.1002/cmdc.202000497
Rational Design of Azastatin as a Potential ADC Payload with Reduced Bystander Killing
Abstract
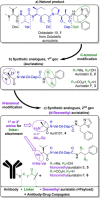
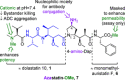
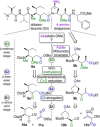
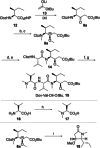
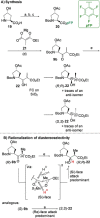
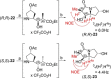
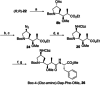
Auristatins are a class of ultrapotent microtubule inhibitors, whose growing clinical popularity in oncology is based upon their use as payloads in antibody-drug conjugates (ADCs). The most widely utilized auristatin, MMAE, has however been shown to cause apoptosis in non-pathological cells proximal to the tumour ("bystander killing"). Herein, we introduce azastatins, a new class of auristatin derivatives encompassing a side chain amine for antibody conjugation. The synthesis of Cbz-azastatin methyl ester, which included the C2-elongation and diastereoselective reduction of two proteinogenic amino acids as key transformations, was accomplished in 22 steps and 0.76 % overall yield. While Cbz-protected azastatin methyl ester (0.13-3.0 nM) inhibited proliferation more potently than MMAE (0.47-6.5 nM), removal of the Cbz-group yielded dramatically increased IC50 -values (9.8-170 nM). We attribute the reduced apparent cytotoxicity of the deprotected azastatin methyl esters to a lack of membrane permeability. These results clearly establish the azastatins as a novel class of cytotoxic payloads ideally suited for use in next-generation ADC development.
Keywords: Antibodies; Cytotoxicity; Diastereoselectivity; Medicinal chemistry; Total synthesis.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
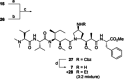
Similar articles
-
Intracellular Released Payload Influences Potency and Bystander-Killing Effects of Antibody-Drug Conjugates in Preclinical Models.Cancer Res. 2016 May 1;76(9):2710-9. doi: 10.1158/0008-5472.CAN-15-1795. Epub 2016 Feb 26. Cancer Res. 2016. PMID: 26921341
-
Design, synthesis and bioactivity evaluation of novel monomethyl auristatin F analogues.Mol Divers. 2025 Feb;29(1):535-550. doi: 10.1007/s11030-024-10873-1. Epub 2024 May 18. Mol Divers. 2025. PMID: 38762686
-
Antibody-Drug Conjugate Payloads; Study of Auristatin Derivatives.Chem Pharm Bull (Tokyo). 2020;68(3):201-211. doi: 10.1248/cpb.c19-00853. Chem Pharm Bull (Tokyo). 2020. PMID: 32115527 Review.
-
Microtubule and tubulin binding and regulation of microtubule dynamics by the antibody drug conjugate (ADC) payload, monomethyl auristatin E (MMAE): Mechanistic insights into MMAE ADC peripheral neuropathy.Toxicol Appl Pharmacol. 2021 Jun 15;421:115534. doi: 10.1016/j.taap.2021.115534. Epub 2021 Apr 20. Toxicol Appl Pharmacol. 2021. PMID: 33852878
-
Quantitative characterization of in vitro bystander effect of antibody-drug conjugates.J Pharmacokinet Pharmacodyn. 2016 Dec;43(6):567-582. doi: 10.1007/s10928-016-9495-8. Epub 2016 Sep 26. J Pharmacokinet Pharmacodyn. 2016. PMID: 27670282 Free PMC article.
Cited by
-
Antibody-drug conjugates: Recent advances in payloads.Acta Pharm Sin B. 2023 Oct;13(10):4025-4059. doi: 10.1016/j.apsb.2023.06.015. Epub 2023 Jun 30. Acta Pharm Sin B. 2023. PMID: 37799390 Free PMC article. Review.
-
The Chemistry Behind ADCs.Pharmaceuticals (Basel). 2021 May 7;14(5):442. doi: 10.3390/ph14050442. Pharmaceuticals (Basel). 2021. PMID: 34067144 Free PMC article. Review.
References
-
- Lee M. D., Dunne T. S., Chang C. C., Siegel M. M., Morton G. O., Ellestad G. A., McGahren W. J., Borders D. B., J. Am. Chem. Soc. 1992, 114, 985–997.
-
- Bose D. S., Thompson A. S., Ching J., Hartley J. A., Berardini M. D., Jenkins T. C., Neidle S., Hurley L. H., Thurston D. E., J. Am. Chem. Soc. 1992, 114, 4939–4941.
-
- Pettit G. R., Kamano Y., Herald C. L., Tuinman A. A., Boettner F. E., Kizu H., Schmidt J. M., Baczynskyi L., Tomer K. B., Bontems R. J., J. Am. Chem. Soc. 1987, 109, 6883–6885.
-
- Bai R., Pettit G. R., Hamel E., J. Biol. Chem. 1990, 265, 17141–17149. - PubMed
-
- Pitot H. C., McElroy E. A., Reid J. M., Windebank A. J., Sloan J. A., Erlichman C., Bagniewski P. G., Walker D. L., Rubin J., Goldberg R. M., et al., Clin. Cancer Res. 1999, 5, 525–531. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

