Broad white matter impairment in multiple system atrophy
- PMID: 33064319
- PMCID: PMC7776008
- DOI: 10.1002/hbm.25227
Broad white matter impairment in multiple system atrophy
Abstract
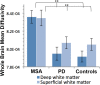
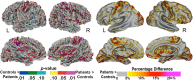
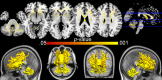
Multiple system atrophy (MSA) is a rare neurodegenerative disorder characterized by the widespread aberrant accumulation of α-synuclein (α-syn). MSA differs from other synucleinopathies such as Parkinson's disease (PD) in that α-syn accumulates primarily in oligodendrocytes, the only source of white matter myelination in the brain. Previous MSA imaging studies have uncovered focal differences in white matter. Here, we sought to build on this work by taking a global perspective on whole brain white matter. In order to do this, in vivo structural imaging and diffusion magnetic resonance imaging were acquired on 26 MSA patients, 26 healthy controls, and 23 PD patients. A refined whole brain approach encompassing the major fiber tracts and the superficial white matter located at the boundary of the cortical mantle was applied. The primary observation was that MSA but not PD patients had whole brain deep and superficial white matter diffusivity abnormalities (p < .001). In addition, in MSA patients, these abnormalities were associated with motor (Unified MSA Rating Scale, Part II) and cognitive functions (Mini-Mental State Examination). The pervasive whole brain abnormalities we observe suggest that there is widespread white matter damage in MSA patients which mirrors the widespread aggregation of α-syn in oligodendrocytes. Importantly, whole brain white matter abnormalities were associated with clinical symptoms, suggesting that white matter impairment may be more central to MSA than previously thought.
Keywords: MRI; diffusion tensor imaging; multiple system atrophy.
© 2020 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
Owen Phillips receives support from BrainKey. Manpreet K. Singh receives research support from Stanford's Child Health Research Institute, National Institute of Mental Health, Office of Research on Women's Health, National Institute of Aging, Neuronetics, Johnson and Johnson, and the Brain and Behavior Foundation. She has been on the advisory board for Sunovion. Olivier Rascol receives funding from Agence Nationale de la Recherche (ANR), CHU de Toulouse, France‐Parkinson, INSERM‐DHOS Recherche Clinique Translationnelle, MJFox Foundation, Programme Hospitalier de Recherche Clinique and European Commission (FP7, H2020). He has also received funding from the International Movement Disorders Society to participate in symposia and contribute to reviews. He is on the advisory board of AbbVie, Adamas, Acorda, Addex, AlzProtect, Apopharma, Astrazeneca, Bial, Biogen, Britannia, Clevexel, INC Reasearch, Lundbeck, Lupin, Merck, MundiPharma, Neuratris, Neuroderm, Novartis, Oxford Biomedica, Parexel, Pfizer, Prexton Therapeutics, Quintiles, Sanofi, Servier, Sunovion, Takeda, Teva, UCB, XenoPort and Zambon. Natalia Del Campo, Françoise Ory‐Magne, Christine Brefel‐Courbon, Monique Galitzky, Claire Thalamas, Katherine L. Narr, Shantanu Joshi, Patrice Péran, and Anne Pavy‐LeTraon have no conflicts of interest to report.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

