Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects
- PMID: 33064340
- PMCID: PMC7675753
- DOI: 10.1002/jmv.26605
Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects
Abstract
Plenty of serologic tests for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been developed so far, thus documenting the importance of evaluating the relevant features of the immune response to this viral agent. The performance of these assays is currently under investigation. Amongst them, LIAISON® SARS-CoV-2 S1/S2 IgG by DiaSorin and Elecsys Anti-SARS-CoV-2 cobas® by Roche are currently used by laboratory medicine hospital departments in Italy and many other countries. In the present study, we firstly compared two serologic tests on serum samples collected at two different time points from 46 laboratory-confirmed coronavirus disease-2019 (COVID-19) subjects. Secondly, 85 negative serum samples collected before the SARS-CoV-2 pandemic were analyzed. Thirdly, possible correlations between antibody levels and the resulting neutralizing activity against a clinical isolate of SARS-CoV-2 were evaluated. Results revealed that both tests are endowed with low sensitivity on the day of hospital admission, which increased to 97.8% and 100% for samples collected after 15 days for DiaSorin and Roche tests, respectively. The specificity evaluated for the two tests ranges from 96.5% to 100%, respectively. Importantly, a poor direct correlation between antibody titers and neutralizing activity levels was evidenced in the present study. These data further shed light on both potentials and possible limitations related to SARS-CoV-2 serology. In this context, great efforts are still necessary for investigating antibody kinetics to develop novel diagnostic algorithms. Moreover, further investigations on the role of neutralizing antibodies and their correlate of protection will be of paramount importance for the development of effective vaccines.
Keywords: COVID-19 diagnostic assays; SARS-CoV-2 serology; neutralizing activity.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
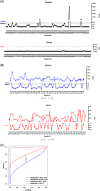
 , T0 solid blue line, T15 dotted blue line) and Roche (
, T0 solid blue line, T15 dotted blue line) and Roche ( , T0 solid red line, T15 dotted red line) tests of sera from subjects with positive nasopharyngeal swabs. C, Roc curves for DiaSorin (T0 solid blue line, T15 dotted blue line) and Roche (T0 solid red line, T15 dotted red line) tests are reported. *p < .001
, T0 solid red line, T15 dotted red line) tests of sera from subjects with positive nasopharyngeal swabs. C, Roc curves for DiaSorin (T0 solid blue line, T15 dotted blue line) and Roche (T0 solid red line, T15 dotted red line) tests are reported. *p < .001
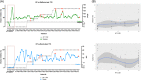
 , grey line). Levels of SARS‐CoV‐2 specific antibodies detected with the tests by Roche and DiaSorin are also indicated (●, green and blue lines). Red dots indicate patients admitted to ICU. B, Spearman correlation analyses between values obtained with the two diagnostic methods and neutralizing activity of sera at 1:100 dilution
, grey line). Levels of SARS‐CoV‐2 specific antibodies detected with the tests by Roche and DiaSorin are also indicated (●, green and blue lines). Red dots indicate patients admitted to ICU. B, Spearman correlation analyses between values obtained with the two diagnostic methods and neutralizing activity of sera at 1:100 dilution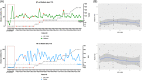
 , grey line). Levels of SARS‐CoV‐2 specific antibodies detected with the tests by Roche and DiaSorin are also indicated (●, green and blue lines). Red dots indicate patients admitted to ICU. B, Spearman correlation analyses between values obtained with the two diagnostic methods and neutralizing activity of sera at 1:200 dilution
, grey line). Levels of SARS‐CoV‐2 specific antibodies detected with the tests by Roche and DiaSorin are also indicated (●, green and blue lines). Red dots indicate patients admitted to ICU. B, Spearman correlation analyses between values obtained with the two diagnostic methods and neutralizing activity of sera at 1:200 dilutionReferences
-
- Long Q, Deng H, Chen J, et al. Antibody responses to SARS‐CoV‐2 in COVID‐19 patients: the perspective application of serological tests in clinical practice. medRxiv. 2020. 10.1101/2020.03.18.20038018 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

