Polymorphisms of the μ-opioid receptor gene influence cerebral pain processing in fibromyalgia
- PMID: 33064887
- PMCID: PMC7821103
- DOI: 10.1002/ejp.1680
Polymorphisms of the μ-opioid receptor gene influence cerebral pain processing in fibromyalgia
Abstract
Background: Dysregulation of the μ-opioid receptor has been reported in fibromyalgia (FM) and was linked to pain severity. Here, we investigated the effect of the functional genetic polymorphism of the μ-opioid receptor gene (OPRM1) (rs1799971) on symptom severity, pain sensitivity and cerebral pain processing in FM subjects and healthy controls (HC).
Methods: Symptom severity and pressure pain sensitivity was assessed in FM subjects (n = 70) and HC (n = 35). Cerebral pain-related activation was assessed by functional magnetic resonance imaging during individually calibrated painful pressure stimuli.
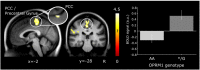
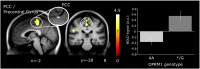
Results: Fibromyalgia subjects were more pain sensitive but no significant differences in pain sensitivity or pain ratings were observed between OPRM1 genotypes. A significant difference was found in cerebral pain processing, with carriers of at least one G-allele showing increased activation in posterior cingulate cortex (PCC) extending to precentral gyrus, compared to AA homozygotes. This effect was significant in FM subjects but not in healthy participants, however, between-group comparisons did not yield significant results. Seed-based functional connectivity analysis was performed with the seed based on differences in PCC/precentral gyrus activation between OPRM1 genotypes during evoked pain across groups. G-allele carriers displayed decreased functional connectivity between PCC/precentral gyrus and prefrontal cortex.
Conclusions: G-allele carriers showed increased activation in PCC/precentral gyrus but decreased functional connectivity with the frontal control network during pressure stimulation, suggesting different pain modulatory processes between OPRM1 genotypes involving altered fronto-parietal network involvement. Furthermore, our results suggest that the overall effects of the OPRM1 G-allele may be driven by FM subjects.
Significance: We show that the functional polymorphism of the μ-opioid receptor gene OPRM1 was associated with alterations in the fronto-parietal network as well as with increased activation of posterior cingulum during evoked pain in FM. Thus, the OPRM1 polymorphism affects cerebral processing in brain regions implicated in salience, attention, and the default mode network. This finding is discussed in the light of pain and the opioid system, providing further evidence for a functional role of OPRM1 in cerebral pain processing.
© 2020 The Authors. European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation -EFIC ®.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


Similar articles
-
The OPRM1 gene and interactions with the 5-HT1a gene regulate conditioned pain modulation in fibromyalgia patients and healthy controls.PLoS One. 2022 Nov 7;17(11):e0277427. doi: 10.1371/journal.pone.0277427. eCollection 2022. PLoS One. 2022. PMID: 36342939 Free PMC article.
-
Gene-to-gene interactions regulate endogenous pain modulation in fibromyalgia patients and healthy controls-antagonistic effects between opioid and serotonin-related genes.Pain. 2017 Jul;158(7):1194-1203. doi: 10.1097/j.pain.0000000000000896. Pain. 2017. PMID: 28282362 Free PMC article.
-
Assessment of opioid receptor μ1 gene A118G polymorphism and its association with pain intensity in patients with fibromyalgia.Rheumatol Int. 2014 Sep;34(9):1257-61. doi: 10.1007/s00296-014-2995-1. Epub 2014 Mar 27. Rheumatol Int. 2014. PMID: 24671502
-
Effects of the OPRM1 A118G Polymorphism (rs1799971) on Opioid Analgesia in Cancer Pain: A Systematic Review and Meta-Analysis.Clin J Pain. 2019 Jan;35(1):77-86. doi: 10.1097/AJP.0000000000000636. Clin J Pain. 2019. PMID: 30028366
-
Meta-analysis of the relevance of the OPRM1 118A>G genetic variant for pain treatment.Pain. 2009 Dec;146(3):270-275. doi: 10.1016/j.pain.2009.07.013. Epub 2009 Aug 14. Pain. 2009. PMID: 19683391 Review.
Cited by
-
Association of Genetic Variants, Such as the μ-Opioid Receptor 1 (OPRM1) rs1799971 and Catechol-O-Methyltransferase (COMT) rs4680, with Phenotypic Expression of Fibromyalgia.Biomedicines. 2025 May 13;13(5):1183. doi: 10.3390/biomedicines13051183. Biomedicines. 2025. PMID: 40427010 Free PMC article.
-
Serotonergic gene-to-gene interaction is associated with mood and GABA concentrations but not with pain-related cerebral processing in fibromyalgia subjects and healthy controls.Mol Brain. 2021 May 12;14(1):81. doi: 10.1186/s13041-021-00789-4. Mol Brain. 2021. PMID: 33980291 Free PMC article.
-
A Comprehensive Review of the Genetic and Epigenetic Contributions to the Development of Fibromyalgia.Biomedicines. 2023 Apr 7;11(4):1119. doi: 10.3390/biomedicines11041119. Biomedicines. 2023. PMID: 37189737 Free PMC article. Review.
-
Fibromyalgia is associated with increased odds of prior pain-precipitated relapse among non-treatment-seeking individuals with opioid use disorder.Ann Med. 2024 Dec;56(1):2422050. doi: 10.1080/07853890.2024.2422050. Epub 2024 Nov 5. Ann Med. 2024. PMID: 39498530 Free PMC article.
-
Effects of functional polymorphisms of opioid receptor mu 1 and catechol-O-methyltransferase on the neural processing of pain.Psychiatry Clin Neurosci. 2024 May;78(5):300-308. doi: 10.1111/pcn.13648. Epub 2024 Feb 25. Psychiatry Clin Neurosci. 2024. PMID: 38403942 Free PMC article.
References
-
- Albrecht, D. S. , Forsberg, A. , Sandström, A. , Bergan, C. , Kadetoff, D. , Protsenko, E. , Lampa, J. , Lee, Y. C. , Höglund, C. O. , Catana, C. , Cervenka, S. , Akeju, O. , Lekander, M. , Cohen, G. , Halldin, C. , Taylor, N. , Kim, M. , Hooker, J. M. , Edwards, R. R. , … Loggia, M. L. (2019). Brain glial activation in fibromyalgia – A multi‐site positron emission tomography investigation. Brain, Behavior, and Immunity, 75, 72–83. 10.1016/j.bbi.2018.09.018 - DOI - PMC - PubMed
-
- Ballina, L. E. , Ulirsch, J. C. , Soward, A. C. , Rossi, C. , Rotolo, S. , Linnstaedt, S. D. , Heafner, T. , Foley, K. A. , Batts, J. , Collette, R. , Holbrook, D. , Zelman, S. , & McLean, S. A. (2013). μ‐Opioid receptor gene A118G polymorphism predicts pain recovery after sexual assault. The Journal of Pain, 14, 165–171. 10.1016/j.jpain.2012.10.013 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

