An Axon-Pathfinding Mechanism Preserves Epithelial Tissue Integrity
- PMID: 33065006
- PMCID: PMC7755670
- DOI: 10.1016/j.cub.2020.09.061
An Axon-Pathfinding Mechanism Preserves Epithelial Tissue Integrity
Abstract
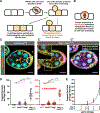
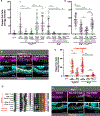
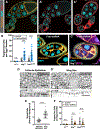
Epithelial tissues form the boundaries of organs, where they perform a range of functions, including secretion, absorption, and protection. These tissues are commonly composed of discrete cell layers-sheets of cells that are one-cell thick. In multiple systems examined, epithelial cells round up and move in the apical direction before dividing, likely in response to neighbor-cell crowding [1-6]. Because of this movement, daughter cells may be born displaced from the tissue layer. Reintegration of these displaced cells supports tissue growth and maintains tissue architecture [4]. Two conserved IgCAMs (immunoglobulin superfamily cell adhesion molecules), neuroglian (Nrg) and fasciclin 2 (Fas2), participate in cell reintegration in the Drosophila follicular epithelium [4]. Like their vertebrate orthologs L1CAM and NCAM1/2, respectively, Nrg and Fas2 are cell adhesion molecules primarily studied in the context of nervous system development [7-10]. Consistent with this, we identify another neural IgCAM, Fasciclin 3 (Fas3), as a reintegration factor. Nrg, Fas2, and Fas3 are components of the insect septate junction, the functional equivalent of the vertebrate tight junction, but proliferating follicle cells do not have mature septate junctions, and we find that the septate junction protein neurexin IV does not participate in reintegration [11, 12]. Here, we show that epithelial reintegration works in the same way as IgCAM-mediated axon growth and pathfinding; it relies not only on extracellular adhesion but also mechanical coupling between IgCAMs and the lateral spectrin-based membrane skeleton. Our work indicates that reintegration is mediated by a distinct epithelial adhesion assembly that is compositionally and functionally equivalent to junctions made between axons.
Keywords: adhesion; epithelia; epithelial junctions; reintegration.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

Comment in
-
Cell Biology: Pardon the Intrusion.Curr Biol. 2020 Dec 21;30(24):R1481-R1484. doi: 10.1016/j.cub.2020.10.036. Curr Biol. 2020. PMID: 33352130
Similar articles
-
Fas2EB112: a tale of two chromosomes.G3 (Bethesda). 2024 May 7;14(5):jkae047. doi: 10.1093/g3journal/jkae047. G3 (Bethesda). 2024. PMID: 38447284 Free PMC article.
-
Lateral adhesion drives reintegration of misplaced cells into epithelial monolayers.Nat Cell Biol. 2015 Nov;17(11):1497-1503. doi: 10.1038/ncb3248. Epub 2015 Sep 28. Nat Cell Biol. 2015. PMID: 26414404 Free PMC article.
-
The lateral mobility of cell adhesion molecules is highly restricted at septate junctions in Drosophila.BMC Cell Biol. 2008 Jul 18;9:38. doi: 10.1186/1471-2121-9-38. BMC Cell Biol. 2008. PMID: 18638384 Free PMC article.
-
Neuronal immunoglobulin superfamily cell adhesion molecules in epithelial morphogenesis: insights from Drosophila.Philos Trans R Soc Lond B Biol Sci. 2020 Oct 12;375(1809):20190553. doi: 10.1098/rstb.2019.0553. Epub 2020 Aug 24. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32829687 Free PMC article. Review.
-
Molecular organization and function of invertebrate occluding junctions.Semin Cell Dev Biol. 2014 Dec;36:186-93. doi: 10.1016/j.semcdb.2014.09.009. Epub 2014 Sep 17. Semin Cell Dev Biol. 2014. PMID: 25239398 Review.
Cited by
-
Insights Into Mechanisms of Oriented Division From Studies in 3D Cellular Models.Front Cell Dev Biol. 2022 Mar 9;10:847801. doi: 10.3389/fcell.2022.847801. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35356279 Free PMC article. Review.
-
A novel tool for the unbiased characterization of epithelial monolayer development in culture.Mol Biol Cell. 2023 Apr 1;34(4):ar25. doi: 10.1091/mbc.E22-04-0121. Epub 2023 Jan 25. Mol Biol Cell. 2023. PMID: 36696175 Free PMC article.
-
Expanding the Junction: New Insights into Non-Occluding Roles for Septate Junction Proteins during Development.J Dev Biol. 2021 Mar 21;9(1):11. doi: 10.3390/jdb9010011. J Dev Biol. 2021. PMID: 33801162 Free PMC article. Review.
-
Fas2EB112: a tale of two chromosomes.G3 (Bethesda). 2024 May 7;14(5):jkae047. doi: 10.1093/g3journal/jkae047. G3 (Bethesda). 2024. PMID: 38447284 Free PMC article.
-
Interaction between Discs large and Pins/LGN/GPSM2: a comparison across species.Biol Open. 2021 Nov 15;10(11):bio058982. doi: 10.1242/bio.058982. Epub 2021 Nov 2. Biol Open. 2021. PMID: 34596678 Free PMC article.
References
-
- Sauer FC (1936). The interkinetic migration of embryonic epithelial nuclei. J. Morphol 60, 1–11.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

