SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates
- PMID: 33065034
- PMCID: PMC7553736
- DOI: 10.1016/S0140-6736(20)32137-1
SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates
Abstract
Understanding immune responses to severe acute respiratory syndrome coronavirus 2 is crucial to understanding disease pathogenesis and the usefulness of bridge therapies, such as hyperimmune globulin and convalescent human plasma, and to developing vaccines, antivirals, and monoclonal antibodies. A mere 11 months ago, the canvas we call COVID-19 was blank. Scientists around the world have worked collaboratively to fill in this blank canvas. In this Review, we discuss what is currently known about human humoral and cellular immune responses to severe acute respiratory syndrome coronavirus 2 and relate this knowledge to the COVID-19 vaccines currently in phase 3 clinical trials.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
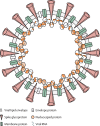

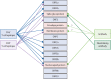
References
-
- Cavanaugh D. Coronaviruses and toroviruses. In: Zuckerman AJ, Banatvala JE, Pattinson JR, Griffiths P, Schoub B, editors. Principles and practice of clinical virology. 5th edn. John Wiley & Sons; London, UK: 2004. pp. 379–397.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

