High-Resolution Cryo-EM Structure of the Cardiac Actomyosin Complex
- PMID: 33065066
- PMCID: PMC7796959
- DOI: 10.1016/j.str.2020.09.013
High-Resolution Cryo-EM Structure of the Cardiac Actomyosin Complex
Abstract
Heart contraction depends on a complicated array of interactions between sarcomeric proteins required to convert chemical energy into mechanical force. Cyclic interactions between actin and myosin molecules, controlled by troponin and tropomyosin, generate the sliding force between the actin-based thin and myosin-based thick filaments. Alterations in this sophisticated system due to missense mutations can lead to cardiovascular diseases. Numerous structural studies proposed pathological mechanisms of missense mutations at the myosin-myosin, actin-tropomyosin, and tropomyosin-troponin interfaces. However, despite the central role of actomyosin interactions a detailed structural description of the cardiac actomyosin interface remained unknown. Here, we report a cryo-EM structure of a cardiac actomyosin complex at 3.8 Å resolution. The structure reveals the molecular basis of cardiac diseases caused by missense mutations in myosin and actin proteins.
Keywords: actin; cardiac muscle; cryoelectron microscopy; myosin.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
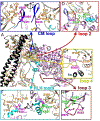

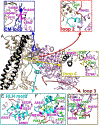

References
-
- Aljeaid D, Sanchez AI, Wakefield E, Chadwell SE, Moore N, Prada CE, and Zhang W (2019). Prevalence of pathogenic and likely pathogenic variants in the RASopathy genes in patients who have had panel testing for cardiomyopathy. Am J Med Genet A 179, 608–614. - PubMed
-
- Augiere C, Megy S, El Malti R, Boland A, El Zein L, Verrier B, Megarbane A, Deleuze JF, and Bouvagnet P (2015). A Novel Alpha Cardiac Actin (ACTC1) Mutation Mapping to a Domain in Close Contact with Myosin Heavy Chain Leads to a Variety of Congenital Heart Defects, Arrhythmia and Possibly Midline Defects. PLoS One 10, e0127903. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

