Biological effects of inhaled hydraulic fracturing sand dust. V. Pulmonary inflammatory, cytotoxic and oxidant effects
- PMID: 33065154
- PMCID: PMC7748298
- DOI: 10.1016/j.taap.2020.115280
Biological effects of inhaled hydraulic fracturing sand dust. V. Pulmonary inflammatory, cytotoxic and oxidant effects
Abstract
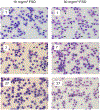
The pulmonary inflammatory response to inhalation exposure to a fracking sand dust (FSD 8) was investigated in a rat model. Adult male Sprague-Dawley rats were exposed by whole-body inhalation to air or an aerosol of a FSD, i.e., FSD 8, at concentrations of 10 or 30 mg/m3, 6 h/d for 4 d. The control and FSD 8-exposed rats were euthanized at post-exposure time intervals of 1, 7 or 27 d and pulmonary inflammatory, cytotoxic and oxidant responses were determined. Deposition of FSD 8 particles was detected in the lungs of all the FSD 8-exposed rats. Analysis of bronchoalveolar lavage parameters of toxicity, oxidant generation, and inflammation did not reveal any significant persistent pulmonary toxicity in the FSD 8-exposed rats. Similarly, the lung histology of the FSD 8-exposed rats showed only minimal changes in influx of macrophages following the exposure. Determination of global gene expression profiles detected statistically significant differential expressions of only six and five genes in the 10 mg/m3, 1-d post-exposure, and the 30 mg/m3, 7-d post-exposure FSD 8 groups, respectively. Taken together, data obtained from the present study demonstrated that FSD 8 inhalation exposure resulted in no statistically significant toxicity or gene expression changes in the lungs of the rats. In the absence of any information about its potential toxicity, a comprehensive rat animal model study (see Fedan, J.S., Toxicol Appl Pharmacol. 000, 000-000, 2020) has been designed to investigate the bioactivities of several FSDs in comparison to MIN-U-SIL® 5, a respirable α-quartz reference dust used in previous animal models of silicosis, in several organ systems.
Keywords: Fracking sand dust; Gene expression; Inflammation; Lung; Pulmonary toxicity; Rats.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest
The authors declare that they have no conflicts of interest in relation to this publication.
Figures
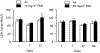

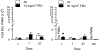
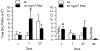
References
-
- Anderson SE, Shane H, Long C, Marrocco A, Lukomska E, Roberts JR, Marshall N, Fedan JS, 2020. Biological effects of inhaled hydraulic fracturing sand dust. VIII. Immunotoxicity. Toxicol. Appl. Pharmacol (Manuscript submitted to this journal as a tandem paper to accompany this manuscript). - PMC - PubMed
-
- Andrews S, 2010. FastQC: A Quality Control Tool for High Throughput Sequence Data http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

