Biological effects of inhaled hydraulic fracturing sand dust. III. Cytotoxicity and pro-inflammatory responses in cultured murine macrophage cells
- PMID: 33065155
- PMCID: PMC7952011
- DOI: 10.1016/j.taap.2020.115281
Biological effects of inhaled hydraulic fracturing sand dust. III. Cytotoxicity and pro-inflammatory responses in cultured murine macrophage cells
Abstract
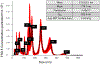
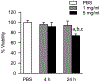
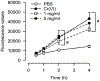
Cultured murine macrophages (RAW 264.7) were used to investigate the effects of fracking sand dust (FSD) for its pro-inflammatory activity, in order to gain insight into the potential toxicity to workers associated with inhalation of FSD during hydraulic fracturing. While the role of respirable crystalline silica in the development of silicosis is well documented, nothing is known about the toxicity of inhaled FSD. The FSD (FSD 8) used in these studies was from an unconventional gas well drilling site. FSD 8was prepared as a 10 mg/ml stock solution in sterile PBS, vortexed for 15 s, and allowed to sit at room temperature for 30 min before applying the suspension to RAW 264.7cells. Compared to PBS controls, cellular viability was significantly decreased after a 24 h exposure to FSD. Intracellular reactive oxygen species (ROS) production and the production of IL-6, TNFα, and endothelin-1 (ET-1) were up-regulated as a result of the exposure, whereas the hydroxyl radical (.OH) was only detected in an acellular system. Immunofluorescent staining of cells against TNFα revealed that FSD 8 caused cellular blebbing, and engulfment of FSD 8 by macrophages was observed with enhanced dark-field microscopy. The observed changes in cellular viability, cellular morphology, free radical generation and cytokine production all confirm that FSD 8 is cytotoxic to RAW 264.7 cells and warrants future studies into the specific pathways and mechanisms by which these toxicities occur.
Keywords: Cytotoxicity; Fracking sand dust; Inflammation; Macrophages; Occupational exposure.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest
The authors of this paper have no conflicts of interest to disclose.
Figures






References
-
- Anderson SE, Shane H, Long C, Marrocco A, Lukomska E, Roberts JR, Marshall N, Fedan JS, 2020. Biological effects of inhaled hydraulic fracturing sand dust. VIII. Immunotoxicity. Toxicol. Appl. Pharmacol (Manuscript submitted to this journal as a tandem paper to accompany this manuscript.). - PMC - PubMed
-
- Azad N, Rojanasakul Y, Vallyathan V, 2008. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J. Toxicol. Environ. Health B Crit. Rev 11, 1–15. - PubMed
-
- Benson ME, Wilson AB, 2015. Frac Sand in the United States - A Geological and Industry Overview. U.S. Geological Survey Open-File Report 2015–1107, 78 p. 10.3133/ofr20151107. - DOI
-
- Borm PJ, Tran L, Donaldson K, 2011. The carcinogenic action of crystalline silica: a review of the evidence supporting secondary inflammation-driven genotoxicity as a principal mechanism. Crit. Rev. Toxicol 41, 756–770. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

