Randomized Trial of 2 Delayed-Release Formulations of Linaclotide in Patients With Irritable Bowel Syndrome With Constipation
- PMID: 33065589
- PMCID: PMC8279899
- DOI: 10.14309/ajg.0000000000000967
Randomized Trial of 2 Delayed-Release Formulations of Linaclotide in Patients With Irritable Bowel Syndrome With Constipation
Abstract
Introduction: Immediate-release (IR) formulation of linaclotide 290 μg improves abdominal pain and constipation (APC) in patients with irritable bowel syndrome (IBS) with constipation. Delayed-release (DR) formulations were developed on the premise that targeting the ileum (delayed-release formulation 1 [DR1]) or ileocecal junction and cecum (MD-7246, formerly DR2) would modulate linaclotide's secretory effects while preserving pain relief effects.
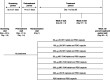
Methods: This phase 2b study randomized patients with IBS with constipation to placebo or 1 of 7 once-daily linaclotide doses (DR1 30, 100, or 300 μg; MD-7246 30, 100, or 300 μg; or IR 290 μg) for 12 weeks. Key efficacy endpoints were change from baseline in abdominal pain and complete spontaneous bowel movement frequency, and 6/12-week combined APC+1 responder rate.
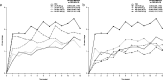
Results: Overall, 532 patients were randomized; mean age was 45.1 years, and most were women (83.3%) and White (64.7%). All linaclotide DR1 and MD-7246 groups experienced greater improvements in abdominal pain from baseline and vs placebo throughout treatment. Linaclotide DR1 and IR led to numerically greater improvements from baseline in complete spontaneous bowel movement frequency and higher APC+1 responder rates compared with placebo; MD-7246 results were similar to placebo. Diarrhea was the most common adverse event with DR1 and IR; rates were similar between MD-7246 and placebo.
Discussion: Altering the site of drug delivery in the intestine might uncouple linaclotide's pain relief from secretory effects. Persistent, modest abdominal pain improvement with limited impact on bowel symptom parameters, as seen across MD-7246 doses, warrants further study of MD-7246 as a novel treatment for abdominal pain, regardless of IBS subtype.
Trial registration: ClinicalTrials.gov NCT02559206.
Copyright © 2020 by The American College of Gastroenterology.
Conflict of interest statement
Figures


References
-
- Drossman DA. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016;150:1262–79. - PubMed
-
- Lacy BE, Mearin F, Chang L et al. Bowel disorders. Gastroenterology 2016;150:1393–407.e5. - PubMed
-
- Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–21. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

