Dysregulated RASGRP1 expression through RUNX1 mediated transcription promotes autoimmunity
- PMID: 33065764
- PMCID: PMC7894479
- DOI: 10.1002/eji.201948451
Dysregulated RASGRP1 expression through RUNX1 mediated transcription promotes autoimmunity
Abstract
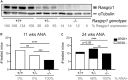
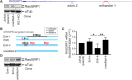
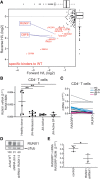
RasGRP1 is a Ras guanine nucleotide exchange factor, and an essential regulator of lymphocyte receptor signaling. In mice, Rasgrp1 deletion results in defective T lymphocyte development. RASGRP1-deficient patients suffer from immune deficiency, and the RASGRP1 gene has been linked to autoimmunity. However, how RasGRP1 levels are regulated, and if RasGRP1 dosage alterations contribute to autoimmunity remains unknown. We demonstrate that diminished Rasgrp1 expression caused defective T lymphocyte selection in C57BL/6 mice, and that the severity of inflammatory disease inversely correlates with Rasgrp1 expression levels. In patients with autoimmunity, active inflammation correlated with decreased RASGRP1 levels in CD4+ T cells. By analyzing H3K27 acetylation profiles in human T cells, we identified a RASGRP1 enhancer that harbors autoimmunity-associated SNPs. CRISPR-Cas9 disruption of this enhancer caused lower RasGRP1 expression, and decreased binding of RUNX1 and CBFB transcription factors. Analyzing patients with autoimmunity, we detected reduced RUNX1 expression in CD4+ T cells. Lastly, we mechanistically link RUNX1 to transcriptional regulation of RASGRP1 to reveal a key circuit regulating RasGRP1 expression, which is vital to prevent inflammatory disease.
Keywords: RASGRP1; RUNX1; T cells; autoimmunity; transcription.
© 2020 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
Jeroen Roose is a co‐founder and scientific advisor of Seal Biosciences, Inc. and on the scientific advisory committee for the Mark Foundation for Cancer research. A.M. is a cofounder, member of the Boards of Directors and a member of the Scientific Advisory Boards of Spotlight Therapeutics and Arsenal Biosciences. A.M. has served as an advisor to Juno Therapeutics, was a member of the scientific advisory board at PACT Pharma, and was an advisor to Trizell. A.M. owns stock in Arsenal Biosciences, Spotlight Therapeutics and PACT Pharma. The Marson lab has received research support from Juno Therapeutics, Epinomics, Sanofi, GlaxoSmithKline, Gilead, and Anthem. D.R.S. is a co‐founder of Beeline Therapeutics. The other authors have no commercial or financial interests.
Figures


References
-
- Dower, N. A. , Stang, S. L. , Bottorff, D. A. , Ebinu, J. O. , Dickie, P. , Ostergaard, H. L. , Stone, J. C. , RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 2000. 1: 317–321. - PubMed
-
- Priatel, J. J. , Teh, S. J. , Dower, N. A. , Stone, J. C. and Teh, H. S. , RasGRP1 transduces low‐grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity 2002. 17: 617–627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

