Macrophage-Derived Iron-Bound Lipocalin-2 Correlates with Renal Recovery Markers Following Sepsis-Induced Kidney Damage
- PMID: 33065981
- PMCID: PMC7589935
- DOI: 10.3390/ijms21207527
Macrophage-Derived Iron-Bound Lipocalin-2 Correlates with Renal Recovery Markers Following Sepsis-Induced Kidney Damage
Abstract
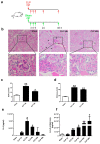
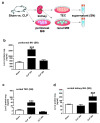
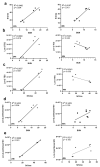
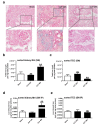
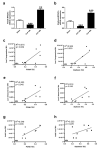
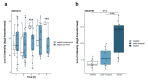
During the course of sepsis in critically ill patients, kidney dysfunction and damage are among the first events of a complex scenario toward multi-organ failure and patient death. Acute kidney injury triggers the release of lipocalin-2 (Lcn-2), which is involved in both renal injury and recovery. Taking into account that Lcn-2 binds and transports iron with high affinity, we aimed at clarifying if Lcn-2 fulfills different biological functions according to its iron-loading status and its cellular source during sepsis-induced kidney failure. We assessed Lcn-2 levels both in serum and in the supernatant of short-term cultured renal macrophages (MΦ) as well as renal tubular epithelial cells (TEC) isolated from either Sham-operated or cecal ligation and puncture (CLP)-treated septic mice. Total kidney iron content was analyzed by Perls' staining, while Lcn-2-bound iron in the supernatants of short-term cultured cells was determined by atomic absorption spectroscopy. Lcn-2 protein in serum was rapidly up-regulated at 6 h after sepsis induction and subsequently increased up to 48 h. Lcn-2-levels in the supernatant of TEC peaked at 24 h and were low at 48 h with no change in its iron-loading. In contrast, in renal MΦ Lcn-2 was low at 24 h, but increased at 48 h, where it mainly appeared in its iron-bound form. Whereas TEC-secreted, iron-free Lcn-2 was associated with renal injury, increased MΦ-released iron-bound Lcn-2 was linked to renal recovery. Therefore, we hypothesized that both the cellular source of Lcn-2 as well as its iron-load crucially adds to its biological function during sepsis-induced renal injury.
Keywords: CLP; iron; lipocalin-2; macrophages; renal tubular epithelial cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kellum J.A., Chawla L.S., Keener C., Singbartl K., Palevsky P.M., Pike F.L., Yealy D.M., Huang D.T., Angus D.C. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am. J. Respir. Crit. Care Med. 2016;193:281–287. doi: 10.1164/rccm.201505-0995OC. - DOI - PMC - PubMed
-
- Shapiro N.I., Trzeciak S., Hollander J.E., Birkhahn R., Otero R., Osborn T.M., Moretti E., Nguyen H.B., Gunnerson K.J., Milzman D., et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit. Care Med. 2009;37:96–104. doi: 10.1097/CCM.0b013e318192fd9d. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

