Inducible Nitric Oxide Regulates Na-Glucose Co-transport in a Spontaneous SAMP1/YitFc Mouse Model of Chronic Ileitis
- PMID: 33065982
- PMCID: PMC7600670
- DOI: 10.3390/nu12103116
Inducible Nitric Oxide Regulates Na-Glucose Co-transport in a Spontaneous SAMP1/YitFc Mouse Model of Chronic Ileitis
Abstract
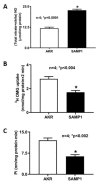
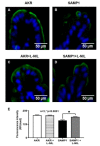
In mammalian small intestine, glucose is primarily absorbed via Na-dependent glucose co-transporter (SGLT1) on the brush border membrane (BBM) of absorptive villus cells. Malabsorption of nutrients (e.g., glucose) leads to malnutrition, a common symptom of inflammatory bowel disease (IBD), where the mucosa is characterized by chronic inflammation. Inducible nitric oxide (iNO) is known to be elevated in IBD mucosa. SAMP1/YitFc (SAMP1) mouse is a spontaneous model of chronic ileitis that develops lesions in its terminal ileum, very similar to human IBD. How SGLT1 may be affected in SAMP1 model of chronic ileitis is unknown. Ten-week-old SAMP1 mice with AKR mice as control were treated with N6-(1-iminoethyl)-L-lysine dihydrochloride (L-NIL) to inhibit iNO production. Intracellular NO levels were found to be increased in villus cells from SAMP1 mice. Moreover, SGLT1 and Na+/K+-ATPase activities and BBM SGLT1 expression were significantly decreased. However, L-NIL treatment reduced the intracellular iNO production, and reversed both downregulated SGLT1 and Na+/K+-ATPase activities in SAMP1 mice. Inhibition of iNO by L-NIL treatment also significantly reversed the BBM SGLT1 protein expression in SAMP1 mice. L-NIL reversed the inflammation mediated downregulation of SGLT1 activity by restoring the BBM SGLT1 expression. Thus, regulation of SGLT1 in chronic ileitis is likely mediated by iNO.
Keywords: L-NIL; SAMP1/YitFc; SGLT1; inducible nitric oxide; inflammatory bowel disease.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures








Similar articles
-
Unique Regulation of Coupled NaCl Absorption by Inducible Nitric Oxide in a Spontaneous SAMP1/YitFc Mouse Model of Chronic Intestinal Inflammation.Inflamm Bowel Dis. 2021 Oct 20;27(11):1804-1812. doi: 10.1093/ibd/izab093. Inflamm Bowel Dis. 2021. PMID: 34019094 Free PMC article.
-
Inhibition of autotaxin alleviates inflammation and increases the expression of sodium-dependent glucose cotransporter 1 and Na+/H+ exchanger 3 in SAMP1/Fc mice.Am J Physiol Gastrointest Liver Physiol. 2018 Nov 1;315(5):G762-G771. doi: 10.1152/ajpgi.00215.2018. Epub 2018 Aug 17. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 30118349 Free PMC article.
-
Chronic and selective inhibition of basolateral membrane Na-K-ATPase uniquely regulates brush border membrane Na absorption in intestinal epithelial cells.Am J Physiol Cell Physiol. 2015 Apr 15;308(8):C650-6. doi: 10.1152/ajpcell.00355.2014. Epub 2015 Feb 4. Am J Physiol Cell Physiol. 2015. PMID: 25652450 Free PMC article.
-
SAMP1/YitFc mouse strain: a spontaneous model of Crohn's disease-like ileitis.Inflamm Bowel Dis. 2011 Dec;17(12):2566-84. doi: 10.1002/ibd.21638. Epub 2011 May 6. Inflamm Bowel Dis. 2011. PMID: 21557393 Free PMC article. Review.
-
Glucose transporters in the small intestine in health and disease.Pflugers Arch. 2020 Sep;472(9):1207-1248. doi: 10.1007/s00424-020-02439-5. Epub 2020 Aug 23. Pflugers Arch. 2020. PMID: 32829466 Free PMC article. Review.
Cited by
-
Unique Regulation of Coupled NaCl Absorption by Inducible Nitric Oxide in a Spontaneous SAMP1/YitFc Mouse Model of Chronic Intestinal Inflammation.Inflamm Bowel Dis. 2021 Oct 20;27(11):1804-1812. doi: 10.1093/ibd/izab093. Inflamm Bowel Dis. 2021. PMID: 34019094 Free PMC article.
-
Empagliflozin attenuates intestinal inflammation through suppression of nitric oxide synthesis and myeloperoxidase activity in in vitro and in vivo models of colitis.Inflammopharmacology. 2024 Feb;32(1):377-392. doi: 10.1007/s10787-023-01227-8. Epub 2023 Apr 22. Inflammopharmacology. 2024. PMID: 37086302 Free PMC article.
-
Intestinal Epithelial Creatine Transporter SLC6A8 Dysregulation in Inflammation and in Response to Adherent Invasive E. coli Infection.Int J Mol Sci. 2024 Jun 13;25(12):6537. doi: 10.3390/ijms25126537. Int J Mol Sci. 2024. PMID: 38928243 Free PMC article.
-
Salmonella re-engineers the intestinal environment to break colonization resistance in the presence of a compositionally intact microbiota.Cell Host Microbe. 2024 Oct 9;32(10):1774-1786.e9. doi: 10.1016/j.chom.2024.07.025. Epub 2024 Aug 23. Cell Host Microbe. 2024. PMID: 39181125
References
-
- Wright E., Loo D., Hirayama B., Turk E. Sugar Absorption. In: Leonard Johnson K.B., Ghishan F., Merchant J., Said H., Wood J., editors. Physiology of Gastrointestinal Tract. 4th ed. Elsevier/Academic Press; San Diego, CA, USA: 2006. pp. 1653–1666.
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C., Chan F.K. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
-
- Kaser A., Lee A.-H., Franke A., Glickman J.N., Zeissig S., Tilg H., Nieuwenhuis E.E., Higgins D.E., Schreiber S., Glimcher L.H. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

