Comparison of Different HILIC Stationary Phases in the Separation of Hemopexin and Immunoglobulin G Glycopeptides and Their Isomers
- PMID: 33065988
- PMCID: PMC7594091
- DOI: 10.3390/molecules25204655
Comparison of Different HILIC Stationary Phases in the Separation of Hemopexin and Immunoglobulin G Glycopeptides and Their Isomers
Abstract
Protein glycosylation analysis is challenging due to the structural variety of complex conjugates. However, chromatographically separating glycans attached to tryptic peptides enables their site-specific characterization. For this purpose, we have shown the importance of selecting a suitable hydrophilic interaction liquid chromatography (HILIC) stationary phase in the separation of glycopeptides and their isomers. Three different HILIC stationary phases, i.e., HALO® penta-HILIC, Glycan ethylene bridged hybrid (BEH) Amide, and ZIC-HILIC, were compared in the separation of complex N-glycopeptides of hemopexin and Immunoglobulin G glycoproteins. The retention time increased with the polarity of the glycans attached to the same peptide backbone in all HILIC columns tested in this study, except for the ZIC-HILIC column when adding sialic acid to the glycan moiety, which caused electrostatic repulsion with the negatively charged sulfobetaine functional group, thereby decreasing retention. The HALO® penta-HILIC column provided the best separation results, and the ZIC-HILIC column the worst. Moreover, we showed the potential of these HILIC columns for the isomeric separation of fucosylated and sialylated glycoforms. Therefore, HILIC is a useful tool for the comprehensive characterization of glycoproteins and their isomers.
Keywords: glycopeptide separation; glycopeptides; glycoproteomics; hydrophilic interaction liquid chromatography; separation of glycopeptide isomers.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures
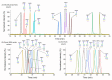

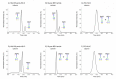
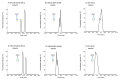
References
-
- Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G., Aebi M., Darvill A.G., Kinoshita T., Packer N.H. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor Laboratory Press; New York, NY, USA: 2017. - PubMed
-
- Lisowska E., Jaskiewicz E. Protein Glycosylation, an overview. eLS. 2012 doi: 10.1002/9780470015902.a0006211.pub3. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

