TMPE Derived from Saffron Natural Monoterpene as Cytotoxic and Multidrug Resistance Reversing Agent in Colon Cancer Cells
- PMID: 33065997
- PMCID: PMC7589963
- DOI: 10.3390/ijms21207529
TMPE Derived from Saffron Natural Monoterpene as Cytotoxic and Multidrug Resistance Reversing Agent in Colon Cancer Cells
Abstract
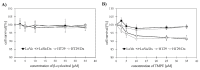
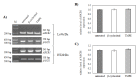
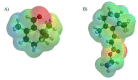
Terpenes constitute one of the largest groups of natural products. They exhibit a wide range of biological activities including antioxidant, anticancer, and drug resistance modulating properties. Saffron extract and its terpene constituents have been demonstrated to be cytotoxic against various types of cancer cells, including breast, liver, lung, pancreatic, and colorectal cancer. In the present work, we have studied anticancer properties of TMPE, a newly synthesized monoterpene derivative of β-cyclocitral-the main volatile produced by the stigmas of unripe crocuses. TMPE presented selective cytotoxic activity to doxorubicin-resistant colon cancer cells and was identified to be an effective MDR modulator in doxorubicin-resistant cancer cells. Synergy between this derivative and doxorubicin was observed. Most probably, TMPE inhibited transport activity of ABCB1 protein without affecting its expression level. Analysis of TMPE physicochemical parameters suggested it was not likely to be transported by ABCB1. Molecular modeling showed TMPE being more reactive molecule than the parental compound-β-cyclocitral. Analysis of electrostatic potential maps of both compounds prompted us to hypothesize that reduced reactivity as well as susceptibility to electrophilic attack were related to the lower general toxicity of β-cyclocitral. All of the above pointed to TMPE as an interesting candidate molecule for MDR reversal in cancer cells.
Keywords: ABCB1 transporter (P-glycoprotein); anticancer activity; colon cancer; monoterpene; multidrug resistance (MDR) reversal; saffron; β-cyclocitral.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Wink M. Plant secondary metabolism: Diversity, function and its evolution. Nat. Prod. Commun. 2008;3:1205–1216. doi: 10.1177/1934578X0800300801. - DOI
-
- Fernandes J. Antitumor monoterpenes. In: de Sousa D., editor. Bioactive Essential Oils and Cancer. Springer International Publishing; Cham, Switzerland: 2015. pp. 175–200.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

