Indications and Outcome in Patients Undergoing Left Atrial Appendage Closure-The Austrian LAAC Registry
- PMID: 33066034
- PMCID: PMC7600032
- DOI: 10.3390/jcm9103274
Indications and Outcome in Patients Undergoing Left Atrial Appendage Closure-The Austrian LAAC Registry
Abstract
Background: Complete real-world data on the indications and outcomes of left atrial appendage closure (LAAC) outside of clinical trials are rare. In this study, we stratified patients undergoing LAAC by indication groups.
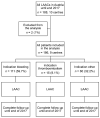
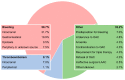
Methods: This analysis of the national multicentre Austrian LAAC Registry comprised all patients that underwent LAAC up until 2018 at the currently active centres in Austria. The baseline characteristics, procedural details and outcomes between the following indication groups were compared: bleeding as an indication for LAAC ("bleeding" group) vs. thromboembolism despite oral anticoagulation (OAC; "thromboembolism" group) vs. an intolerance to OAC for reasons other than the above ("other" group).
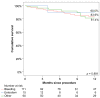
Results: The analysis included 186 patients, with 59.7% in the "bleeding" group, 8.1% in the "thromboembolism" group and 32.2% in the "other" group. The CHADS2 score was the highest in the "thromboembolism" group and the HAS-BLED score was the highest in the "bleeding" group. The procedural outcomes were similar between groups (implantation success, 97.3%), with major complications occurring in 7.0% of patients. One-year survival free from stroke, bleeding or LAAC-associated hospitalisation was 83.9%, 90.0% and 81.4% in the "bleeding", "thromboembolism" and "other" groups, respectively (p = 0.891).
Conclusions: In routine clinical practice, LAAC was used in a heterogeneous patient population with atrial fibrillation (AF) and contraindication, inefficacy or intolerance to OAC. The long-term outcome was favourable in all groups.
Keywords: atrial fibrillation; bleeding; left atrial appendage; registry; stroke.
Conflict of interest statement
H.G. serves as a consultant for Abbott. G.S. received speaker honoraria from Bayer, Biotronik and Boston Scientific. R.K.B. serves as proctor for Boston Scientific and has received grants from Abbott. D.S. received speaker honoraria from Boston Scientific. All other authors have nothing to disclose with respect to this publication.
Figures
References
-
- Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B., Castella M., Diener H.C., Heidbuchel H., Hendriks J., et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. - DOI - PubMed
-
- Meschia J.F., Bushnell C., Boden-Albala B., Braun L.T., Bravata D.M., Chaturvedi S., Creager M.A., Eckel R.H., Elkind M.S., Fornage M., et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. - DOI - PMC - PubMed
-
- Boersma L.V., Schmidt B., Betts T.R., Sievert H., Tamburino C., Teiger E., Pokushalov E., Kische S., Schmitz T., Stein K.M., et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: Peri-procedural outcomes from the EWOLUTION registry. Eur. Heart J. 2016;37:2465–2474. doi: 10.1093/eurheartj/ehv730. - DOI - PMC - PubMed
-
- Lebhart G., Neustädter C., Kytir J. The new Population Register at Statistics Austria: Conceptualization and Methodology for Register-based Flow and Stock Statistics. Austrian J. Stat. 2007;36:277–289. doi: 10.17713/ajs.v36i4.339. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

