Sulfamethoxazole Removal from Drinking Water by Activated Carbon: Kinetics and Diffusion Process
- PMID: 33066051
- PMCID: PMC7587352
- DOI: 10.3390/molecules25204656
Sulfamethoxazole Removal from Drinking Water by Activated Carbon: Kinetics and Diffusion Process
Abstract
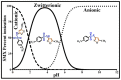
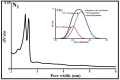
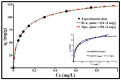
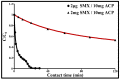
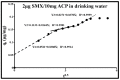
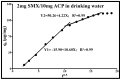
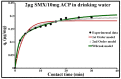
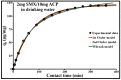
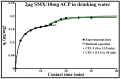
Sulfamethoxazole (SMX), a pharmaceutical residue, which is persistent and mobile in soils, shows low biodegradability, and is frequently found in the different aquatic compartments, can be found at very low concentrations in water intended for human consumption. In conditions compatible with industrial practices, the kinetic reactivity and performance of tap water purification using activated carbon powder (ACP) are examined here using two extreme mass ratios of SMX to ACP: 2 µg/L and 2 mg/L of SMX for only 10 mg/L of ACP. In response to surface chemistry, ACP texture and the intrinsic properties of SMX in water at a pH of 8.1, four kinetic models, and two monosolute equilibrium models showed a total purification of the 2 µg/L of SMX, the presence of energetic heterogeneity of surface adsorption of ACP, rapid kinetics compatible with the residence times of industrial water treatment processes, and kinetics affected by intraparticle diffusion. The adsorption mechanisms proposed are physical mechanisms based mainly on π-π dispersion interactions and electrostatic interactions by SMX-/Divalent cation/ArO- and SMX-/Divalent cation/ArCOO- bridging. Adsorption in tap water, also an innovative element of this study, shows that ACP is very efficient for the purification of very slightly polluted water.
Keywords: activated carbon; diffusion; kinetic; micropollutants; pharmaceuticals; sulfamethoxazole; wastewater; water treatment.
Conflict of interest statement
The author declare no conflict of interest.
Figures
Similar articles
-
Adsorption of pharmaceuticals from biologically treated municipal wastewater using paper mill sludge-based activated carbon.Environ Sci Pollut Res Int. 2019 May;26(13):13173-13184. doi: 10.1007/s11356-019-04823-w. Epub 2019 Mar 21. Environ Sci Pollut Res Int. 2019. PMID: 30903474
-
Super-fine powdered activated carbon (SPAC) for efficient removal of micropollutants from wastewater treatment plant effluent.Water Res. 2016 Mar 1;90:90-99. doi: 10.1016/j.watres.2015.12.001. Epub 2015 Dec 10. Water Res. 2016. PMID: 26724443
-
Magnetic activated carbon for improving the removal of antibiotics by heterogeneous solar photo-Fenton at circumneutral pH.Water Res. 2025 Aug 1;281:123679. doi: 10.1016/j.watres.2025.123679. Epub 2025 Apr 19. Water Res. 2025. PMID: 40294504
-
Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater.Environ Pollut. 2020 Mar;258:113809. doi: 10.1016/j.envpol.2019.113809. Epub 2019 Dec 16. Environ Pollut. 2020. PMID: 31864923
-
Competitive adsorption of tylosin, sulfamethoxazole and Cu(II) on nano-hydroxyapatitemodified biochar in water.Chemosphere. 2020 Feb;240:124884. doi: 10.1016/j.chemosphere.2019.124884. Epub 2019 Sep 16. Chemosphere. 2020. PMID: 31542586
Cited by
-
Response Methodology Optimization and Artificial Neural Network Modeling for the Removal of Sulfamethoxazole Using an Ozone-Electrocoagulation Hybrid Process.Molecules. 2023 Jun 29;28(13):5119. doi: 10.3390/molecules28135119. Molecules. 2023. PMID: 37446780 Free PMC article.
-
Zirconium ferrite incorporated zeolitic imidazolate framework-8: a suitable photocatalyst for degradation of dopamine and sulfamethoxazole in aqueous solution.RSC Adv. 2023 Mar 23;13(14):9563-9575. doi: 10.1039/d3ra01055d. eCollection 2023 Mar 20. RSC Adv. 2023. PMID: 36968036 Free PMC article.
-
Improved methodology to survey veterinary antibiotics in environmental samples using µSPEed microextraction followed by ultraperformance liquid chromatography.Commun Chem. 2025 Mar 8;8(1):68. doi: 10.1038/s42004-025-01454-w. Commun Chem. 2025. PMID: 40057578 Free PMC article.
-
Design of Molecular Logic Gates Via pH-dependent Absorption and Fluorescence Transitions in Drug Molecules.J Fluoresc. 2025 May 27. doi: 10.1007/s10895-025-04375-y. Online ahead of print. J Fluoresc. 2025. PMID: 40423929
-
Pharmacometabolomics Study of Sulfamethoxazole and Trimethoprim in Kidney Transplant Recipients: Real-World Metabolism and Urinary Excretion.Metabolites. 2025 Jul 11;15(7):473. doi: 10.3390/metabo15070473. Metabolites. 2025. PMID: 40710573 Free PMC article.
References
-
- Verlicchi P., Galletti A., Petrovic M., Barceló D. Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J. Hydrol. 2010;389:416–428. doi: 10.1016/j.jhydrol.2010.06.005. - DOI
-
- Furlong E.T., Batt A.L., Glassmeyer S.T., Noriega M.C., Kolpin D., Mash H., Schenck K.M. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States: Pharmaceuticals. Sci. Total. Environ. 2017;579:1629–1642. doi: 10.1016/j.scitotenv.2016.03.128. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

