How Do We Monitor Oxygenation during the Management of PPHN? Alveolar, Arterial, Mixed Venous Oxygen Tension or Peripheral Saturation?
- PMID: 33066076
- PMCID: PMC7600440
- DOI: 10.3390/children7100180
How Do We Monitor Oxygenation during the Management of PPHN? Alveolar, Arterial, Mixed Venous Oxygen Tension or Peripheral Saturation?
Abstract
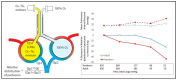
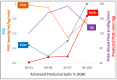
Oxygen is a pulmonary vasodilator and plays an important role in mediating circulatory transition from fetal to postnatal period. Oxygen tension (PO2) in the alveolus (PAO2) and pulmonary artery (PaO2) are the main factors that influence hypoxic pulmonary vasoconstriction (HPV). Inability to achieve adequate pulmonary vasodilation at birth leads to persistent pulmonary hypertension of the newborn (PPHN). Supplemental oxygen therapy is the mainstay of PPHN management. However, optimal monitoring and targeting of oxygenation to achieve low pulmonary vascular resistance (PVR) and optimizing oxygen delivery to vital organs remains unknown. Noninvasive pulse oximetry measures peripheral saturations (SpO2) and a target range of 91-95% are recommended during acute PPHN management. However, for a given SpO2, there is wide variability in arterial PaO2, especially with variations in hemoglobin type (HbF or HbA due to transfusions), pH and body temperature. This review evaluates the role of alveolar, preductal, postductal, mixed venous PO2, and SpO2 in the management of PPHN. Translational and clinical studies suggest maintaining a PaO2 of 50-80 mmHg decreases PVR and augments pulmonary vasodilator management. Nevertheless, there are no randomized clinical trials evaluating outcomes in PPHN targeting SpO2 or PO2. Also, most critically ill patients have umbilical arterial catheters and postductal PaO2 may not be an accurate assessment of oxygen delivery to vital organs or factors influencing HPV. The mixed venous oxygen tension from umbilical venous catheter blood gas may assess pulmonary arterial PO2 and potentially predict HPV. It is crucial to conduct randomized controlled studies with different PO2/SpO2 target ranges for the management of PPHN and compare outcomes.
Keywords: PPHN; oxygen tension; oxygenation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Abman S.H., Hansmann G., Archer S.L., Ivy D.D., Adatia I., Chung W.K., Hanna B.D., Rosenzweig E.B., Raj J.U., Cornfield D., et al. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–2099. doi: 10.1161/CIR.0000000000000329. - DOI - PubMed
-
- Halliday H., Hirschfeld S., Riggs T., Liebman J., Fanaroff A., Bormuth C. Respiratory distress syndrome: Echocardiographic assessment of cardiovascular function and pulmonary vascular resistance. Pediatrics. 1977;60:444–449. - PubMed
-
- Walther F.J., Benders M.J., Leighton J.O. Persistent pulmonary hypertension in premature neonates with severe respiratory distress syndrome. Pediatrics. 1992;90:899–904. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

