Preliminary Study on β3-Adrenoreceptor as Predictor Marker of Relapse in Ewing Sarcoma Patients
- PMID: 33066095
- PMCID: PMC7600453
- DOI: 10.3390/biomedicines8100413
Preliminary Study on β3-Adrenoreceptor as Predictor Marker of Relapse in Ewing Sarcoma Patients
Abstract
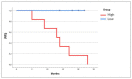
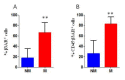
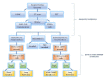
Ewing sarcoma (EWS) is a paediatric aggressive malignant tumour of bones and soft tissues. Multidisciplinary chemotherapies, surgical resection, and radiation represent the only strategies counteracting the disease, however spreading and relapse of disease still remain a clinical issue. Circulating tumour cells (CTCs) are an important feature of EWS but the prognostic significance has not been, yet, clarified. CTCs have been found both in patients with localized disease and in those who recur or metastasize. The identification of markers that can detect recurrences and metastasis remains an important challenge for research. Unfortunately, even most of patients with localized cancer relapsed and the reason has not yet been fully understood. In this clinical study on EWS patients, we evaluated the expression of CD99 antigen and beta-3 adrenergic receptor (β3-AR) on CTCs and bioptic derived cells by flow cytometry. The preliminary data revealed a higher β3-AR expression on cells derived from metastatic or relapsed patients, suggesting a role for the β3-AR as a possible predictive maker of disease recurrence in both patients with metastatic and localized disease.
Keywords: circulating tumour cells; ewing sarcoma; β3-adrenergic receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials

