Effects of Bifidobacterium animalis Subsp. lactis (BPL1) Supplementation in Children and Adolescents with Prader-Willi Syndrome: A Randomized Crossover Trial
- PMID: 33066107
- PMCID: PMC7650793
- DOI: 10.3390/nu12103123
Effects of Bifidobacterium animalis Subsp. lactis (BPL1) Supplementation in Children and Adolescents with Prader-Willi Syndrome: A Randomized Crossover Trial
Abstract
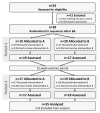
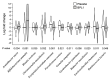
Prader-Willi syndrome (PWS) is a rare genetic disorder characterized by a wide range of clinical manifestations, including obesity, hyperphagia, and behavioral problems. Bifidobacterium animalis subsp. lactis strain BPL1 has been shown to improve central adiposity in adults with simple obesity. To evaluate BPL1's effects in children with PWS, we performed a randomized crossover trial among 39 patients (mean age 10.4 years). Participants were randomized to placebo-BPL1 (n = 19) or BPL1-placebo (n = 20) sequences and underwent a 12-week period with placebo/BPL1 treatments, a 12-week washout period, and a 12-week period with the crossover treatment. Thirty-five subjects completed the study. The main outcome was changes in adiposity, measured by dual-energy X-ray absorptiometry. Secondary outcomes included lipid and glucose metabolism, hyperphagia, and mental health symptoms. Generalized linear modeling was applied to assess differences between treatments. While BPL1 did not modify total fat mass compared to placebo, BPL1 decreased abdominal adiposity in a subgroup of patients older than 4.5 years (n = 28). BPL1 improved fasting insulin concentration and insulin sensitivity. Furthermore, we observed modest improvements in some mental health symptoms. A follow-up trial with a longer treatment period is warranted to determine whether BPL1 supplementation can provide a long-term therapeutic approach for children with PWS (ClinicalTrials.gov NCT03548480).
Keywords: Prader–Willi syndrome; gut microbiota; hyperphagia; insulin sensitivity; mental health; obesity; probiotic supplementation.
Conflict of interest statement
Eric Climent (E.C.), Empar Chenoll (E.C.), and Daniel Ramón (D.R.) are employees of Archer Daniels Midland Co-Biopolis (Valencia, Spain). The rest of the authors declare no conflict of interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

