Exploring the Use of Helicogenic Amino Acids for Optimising Single Chain Relaxin-3 Peptide Agonists
- PMID: 33066369
- PMCID: PMC7602263
- DOI: 10.3390/biomedicines8100415
Exploring the Use of Helicogenic Amino Acids for Optimising Single Chain Relaxin-3 Peptide Agonists
Abstract
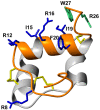
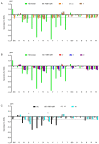
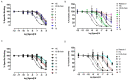
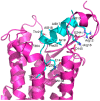
Relaxin-3 is a highly conserved two-chain neuropeptide that acts through its endogenous receptor the Relaxin Family Peptide-3 (RXFP3) receptor. The ligand/receptor system is known to modulate several physiological processes, with changes in food intake and anxiety-levels the most well studied in rodent models. Agonist and antagonist analogues based on the native two-chain peptide are costly to synthesise and not ideal drug leads. Since RXFP3 interacting residues are found in the relaxin B-chain only, this has been the focus of analogue development. The B-chain is unstructured without the A-chain support, but in single-chain variants structure can be induced by dicarba-based helical stapling strategies. Here we investigated whether alternative helical inducing strategies also can enhance structure and activity at RXFP3. Combinations of the helix inducing α-aminoisobutyric acid (Aib) were incorporated into the sequence of the relaxin-3 B-chain. Aib residues at positions 13, 17 and 18 partially reintroduce helicity and activity of the relaxin-3 B-chain, but other positions are generally not suited for modifications. We identify Thr21 as a putative new receptor contact residue important for RXFP3 binding. Cysteine residues were also incorporated into the sequence and cross-linked with dichloroacetone or α, α'-dibromo-m-xylene. However, in contrast to previously reported dicarba variants, neither were found to promote structure and RXFP3 activity.
Keywords: RXFP3; helical stapling; relaxin-3; α-aminoisobutyric acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Binding conformation and determinants of a single-chain peptide antagonist at the relaxin-3 receptor RXFP3.J Biol Chem. 2018 Oct 12;293(41):15765-15776. doi: 10.1074/jbc.RA118.002611. Epub 2018 Aug 21. J Biol Chem. 2018. PMID: 30131342 Free PMC article.
-
Hydrocarbon stapled B chain analogues of relaxin-3 retain biological activity.Peptides. 2016 Oct;84:44-57. doi: 10.1016/j.peptides.2016.08.001. Epub 2016 Aug 3. Peptides. 2016. PMID: 27498038
-
Development of Relaxin-3 Agonists and Antagonists Based on Grafted Disulfide-Stabilized Scaffolds.Front Chem. 2020 Feb 18;8:87. doi: 10.3389/fchem.2020.00087. eCollection 2020. Front Chem. 2020. PMID: 32133341 Free PMC article.
-
Increased feeding and body weight gain in rats after acute and chronic activation of RXFP3 by relaxin-3 and receptor-selective peptides: functional and therapeutic implications.Behav Pharmacol. 2012 Sep;23(5-6):516-25. doi: 10.1097/FBP.0b013e3283576999. Behav Pharmacol. 2012. PMID: 22854307 Review.
-
Relaxin-3/insulin-like peptide 7, a neuropeptide involved in the stress response and food intake.FEBS J. 2010 Dec;277(24):4990-7. doi: 10.1111/j.1742-4658.2010.07931.x. FEBS J. 2010. PMID: 21126312 Review.
References
-
- Ma S., Bonaventure P., Ferraro T., Shen P.J., Burazin T.C., Bathgate R.A., Liu C., Tregear G.W., Sutton S.W., Gundlach A.L. Relaxin-3 in GABA projection neurons of nucleus incertus suggests widespread influence on forebrain circuits via G-protein-coupled receptor-135 in the rat. Neuroscience. 2007;144:165–190. doi: 10.1016/j.neuroscience.2006.08.072. - DOI - PubMed
-
- Sutton S.W., Bonaventure P., Kuei C., Roland B., Chen J., Nepomuceno D., Lovenberg T.W., Liu C. Distribution of G-protein-coupled receptor (GPCR)135 binding sites and receptor mRNA in the rat brain suggests a role for relaxin-3 in neuroendocrine and sensory processing. Neuroendocrinology. 2004;80:298–307. doi: 10.1159/000083656. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

