Intimate Relations-Mitochondria and Ageing
- PMID: 33066461
- PMCID: PMC7589147
- DOI: 10.3390/ijms21207580
Intimate Relations-Mitochondria and Ageing
Abstract
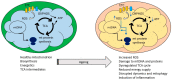
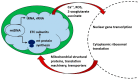
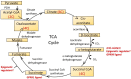
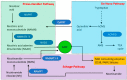
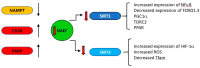
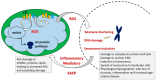
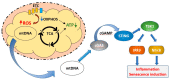
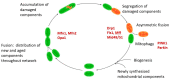
Mitochondrial dysfunction is associated with ageing, but the detailed causal relationship between the two is still unclear. We review the major phenomenological manifestations of mitochondrial age-related dysfunction including biochemical, regulatory and energetic features. We conclude that the complexity of these processes and their inter-relationships are still not fully understood and at this point it seems unlikely that a single linear cause and effect relationship between any specific aspect of mitochondrial biology and ageing can be established in either direction.
Keywords: ROS; ageing; energetics; gene regulation; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

