Carbohydrate Immune Adjuvants in Subunit Vaccines
- PMID: 33066594
- PMCID: PMC7602499
- DOI: 10.3390/pharmaceutics12100965
Carbohydrate Immune Adjuvants in Subunit Vaccines
Abstract
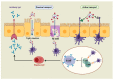
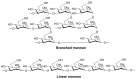
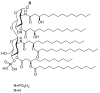
Modern subunit vaccines are composed of antigens and a delivery system and/or adjuvant (immune stimulator) that triggers the desired immune responses. Adjuvants mimic pathogen-associated molecular patterns (PAMPs) that are typically associated with infections. Carbohydrates displayed on the surface of pathogens are often recognized as PAMPs by receptors on antigen-presenting cells (APCs). Consequently, carbohydrates and their analogues have been used as adjuvants and delivery systems to promote antigen transport to APCs. Carbohydrates are biocompatible, usually nontoxic, biodegradable, and some are mucoadhesive. As such, carbohydrates and their derivatives have been intensively explored for the development of new adjuvants. This review assesses the immunological functions of carbohydrate ligands and their ability to enhance systemic and mucosal immune responses against co-administered antigens. The role of carbohydrate-based adjuvants/delivery systems in the development of subunit vaccines is discussed in detail.
Keywords: adjuvants; carbohydrates; immunostimulation; peptide/protein subunit vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Skwarczynski M., Zaman M., Toth I. Lipo-peptides/saccharides for peptide vaccine delivery. In: Kastin A.J., editor. Handbook of Biologically Active Peptides. 2nd ed. Vol. 78. Academic Press; Boston, MA, USA: 2013. pp. 571–579. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

